Rhodium
Synonym(s):Rh/Al2O3;Rhodium;Rhodium black;
- CAS NO.:7440-16-6
- Empirical Formula: Rh
- Molecular Weight: 102.91
- MDL number: MFCD00011201
- EINECS: 231-125-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
What is Rhodium?
Description
Rhodium is one of the platinum group elements, and is found
at very low concentrations in the Earths crust. Rhodium was
discovered by William Hyde Wollaston (England) in 1804. The
origin of the name comes from the Greek word rhodon,
meaning rose. The plated solid is very corrosion resistant and
exceptionally hard. While inert in air and acids, it can produce
a violent reaction to chlorine, bromine pentafluoride, bromine
trifluoride, and fluorine monoxide.
Chemical properties
Rhodium is a rare metal characterised by its silvery-white, lustrous appearance and hard texture. It resists corrosion by air and water at temperatures up to 875 K. It exhibits high reflectivity to light and is soluble in diethyl ether, ethanol and water.
Physical properties
Rhodium is a hard shiny-white metal that resists corrosion from oxygen, moisture, andacids at room temperatures. As a member of group 8 (VIII), 45Rh shares many chemical andphysical properties with cobalt (27Co) just above it and iridium (77Ir) below it in the verticalgroup. Therefore, it is considered one of the elements that are transitory between metals andnonmetals. It is rare and only found in combination with platinum ores.
Rhodium’s melting point is 1,966°C, its boiling point is 3,727°C, and its density is 12.41g/cm3.
Isotopes
There are 52 isotopes of rhodium, ranging from Rh-89 to Rh-122. All are producedartificially with relatively short half-lives except one stable isotope, Rh-103, whichconstitutes 100% of the element’s existence in the Earth’s crust.
Origin of Name
Named after the Greek word rhodon, which means “rose,” because of the reddish color of its salt compounds.
Occurrence
Rhodium is rare, but not as rare as ruthenium. It makes up only 1 part in 20 million of theelements found in the Earth’s crust. Even so, it is considered the 79th most abundant elementand is found mixed with platinum ore, and to a lesser extent, it is found with copper andnickel ores. It is found in Siberia, South Africa, and Ontario, Canada.
Rhodium is recovered from platinum and other ores by refining and purification processesthat start by dissolving the other platinum group metals and related impurities with strongacids that do not affect the rhodium itself. Any remaining platinum group elements areremoved by oxidation and bathing the mixture in chlorine and ammonia.
Rhodium is usually produced as a powder and can be formed by either casting or powdermetallurgy.
History
Rhodium was discovered in 1803 by William Wollaston. He collaborated with Smithson Tennant in a commercial venture, part of which was to produce pure platinum for sale. The first step in the process was to dissolve ordinary platinum in aqua regia (nitric acid + hydrochloric acid). Not all of it went into solution and it left behind a black residue. (Tennant investigated this residue and from it he eventually isolated osmium and iridium.) Wollaston concentrated on the solution of dissolved platinum which also contained palladium. He removed these metals by precipitation and was left with a beautiful red solution from which he obtained rose red crystals. These were sodium rhodium chloride, Na3RhCl6. From them he eventually produced a sample of the metal itself.
Characteristics
Rhodium is one of the six platinum transition elements that include Ru, Rh, Pd, Os, Ir, andPt. Of these metals, rhodium has the highest electrical and thermal conductivity. Although arelatively scarce metal, rhodium makes an excellent electroplated surface that is hard, wearswell, and is permanently bright—ideal for plating the reflectors in automobile headlights.
The Uses of Rhodium
Rhodium is primarily used in automotive catalytic converters (80%). It reduces nitrogen oxides in exhaust gases. It also serves as a catalyst in the chemical industry for producing nitric acid, acetic acid, and hydrogenation reactions. Moreover, it resists atmospheric corrosion and tarnishing at room temperature, making it ideal for electroplating onto metal objects and subsequent polishing. This provides a permanent, attractive finish for jewellery and decorative items. Rhodium is also used to coat optical fibres and mirrors, as well as crucibles, thermocouple elements, and headlamp reflectors.
What are the applications of Application
Rhodium nanoparticles entrapped in aluminum hydroxide matrix preparation is a rhodium catalyst for research
Definition
Metallic element having atomic number 45, group VIII of the periodic table, aw 102.9055, no isotopes, valence = 3.
Production Methods
Pure rhodium is prepared by the reduction of its ammonium salt (dichloropentaaminorhodium).
General Description
This product has been enhanced for energy efficiency.
Hazard
The powder and dust of rhodium metal are flammable in air. Some of the compounds maycause skin irritations. It is best to use approved laboratory procedures when handling any ofthe six elements in the platinum family of metals.
Health Hazard
There are no data demonstrating acute or chronic rhodium-related diseases; irritation and sensitization have occasionally been reported in humans from exposure to the salts of rhodium. Solutions of insoluble salts splashed in the eye may cause mild irritation.
Flammability and Explosibility
Not classified
Industrial uses
Metallic rhodium is the whitest of the platinum metals and does not tarnish under atmospheric conditions. It is insoluble in most acids, including aqua regia, but is attacked by chlorine at elevated temperatures and by hot fuming sulfuric acid. Liquid rhodium dissolves oxygen, and ingots are made by argon-arc melting. At temperatures above 1200 C, rhodium reacts with oxygen to form rhodium oxide, Rh2O3. Rhodium is used to make the nibs of writing pens, to make resistance windings in high-temperature furnaces, for high-temperature thermocouples, as a catalyst, and for laboratory dishes. It is the hardest of the platinum-group metals; the annealed metal has a Brinell hardness of 135. Rhodium is also valued for electroplating jewelry, electric contacts, hospital and surgical instruments, and especially reflectors.
The most important alloys of rhodium are rhodium platinum. They form solid solutions in any proportion, but alloys of more than 40% rhodium are rare. Rhodium is not a potent hardener of platinum but increases its high-temperature strength. It is easily workable and does not tarnish or oxidize at high temperatures. These alloys are used for thermocouples and in the glass industry.
Safety Profile
Handle carefully. It may be a sensitizer but not to the same extent as platinum. Most rholum compounds have only moderate toxicity by ingestion. Flammable when exposed to heat or flame. Violent reaction with chlorine, bromine pentafluoride, bromine trifluoride, and OF2. A catalytic metal
Potential Exposure
Rhodium has few applications by itself, as in rhodium plating of white gold jewelry or plat- ing of electrical parts, such as commutator slip rings, but, mainly, rhodium is used as a component of platinum alloys. Rhodium-containing catalysts have been proposed for use in automotive catalytic converters for exhaust gas cleanup.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Ifmetal fume fever develops, it may last less than 36 h.Note to physician: In case of fume inhalation, treat for pulmonary edema. Give prednisone or other corticosteroidorally to reduce tissue response to fume. Positive-pressureventilation may be necessary. Treat metal fume fever withbed rest, analgesics, and antipyretics.
Carcinogenicity
Chick embryos exposed to rhodium on the eighth day of incubation were stunted; mild reduction of limb size and feather growth inhibition were also observed. A number of rhodium compounds have tested positive in bacterial assays for genetic altering capability.
Environmental Fate
The most common route by which rhodium enters the environment
is as a component of automobile exhaust resulting
from use of catalytic converters. Rhodium is insoluble in water
and all acids, with the exception that very finely separated
material may be dissolved in concentrated sulfuric acid and
aqua regia.
Being largely inert, rhodium can undergo long-range
transport, and particulate phase matter generally leaves the
atmosphere by wet or dry deposition. In an aqueous environment,
rhodium can form complexes with halide and
nitrogen donor ligands, which may be water soluble, but
reactions can be dictated by pH, redox potential, and what
material is available for creating ligands. Reactions in soil can
depend on these same factors, as well as chloride concentrations, and rhodium is seen to be mobile only in
highly acidic soils.
Rhodium has been seen to bioaccumulate in both fresh and
salt water species, and has the potential to biomagnify.
Storage
Color Code—Red (powder): FlammabilityHazard: Store in a flammable materials storage area. Priorto working with this material you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from strong oxidizers and sources of ignition. Where possible, automaticallytransfer material from drums or other storage containers toprocess containers. Sources of ignition, such as smokingand open flames, are prohibited where this chemical is handled, used, or stored. Metal containers involving the transferof this chemical should be grounded and bonded. Whereverthis chemical is used, handled, manufactured, or stored, useexplosion-proof electrical equipment and fittings.
Shipping
Flammable powder, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Toxicity evaluation
Enzymes that have essential sulfhydryl groups in or near the activation sites are completely inhibited. It is thought that rhodium compounds bond to DNA, RNA, or the corresponding purines and inhibit their synthesis.
Incompatibilities
Flammable as a dust, fume, or powder may form explosive mixture with air. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanga- nates, perchlorates, chlorine, bromine, fluorine, etc.); con- tact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, bromine pentafluoride, and bromine trifluoride; chlorine trifluoride; oxygen difluoride.
Waste Disposal
Recovery in view of the high economic value. Recovery techniques for recycling of rhodium in plating wastes and spent catalysts have been described in the literature.
Properties of Rhodium
| Melting point: | 1966 °C (lit.) |
| Boiling point: | 3727 °C (lit.) |
| Density | 12.41 g/cm3 (lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | insoluble in acid solutions, slightly soluble in aqua regia |
| form | wire |
| Specific Gravity | 12.41 |
| color | Red |
| Resistivity | 4.33 μΩ-cm, 20°C |
| Water Solubility | Insoluble |
| Merck | 14,8186 |
| Exposure limits | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| CAS DataBase Reference | 7440-16-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Rhodium(7440-16-6) |
| EPA Substance Registry System | Rhodium (7440-16-6) |
Safety information for Rhodium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Rhodium
Rhodium manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
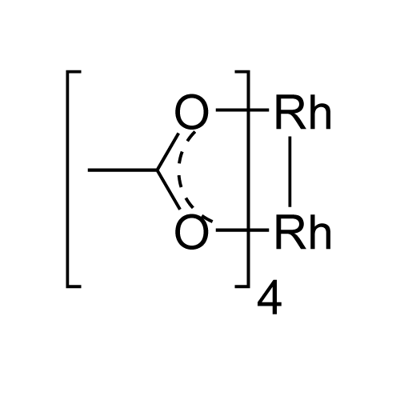
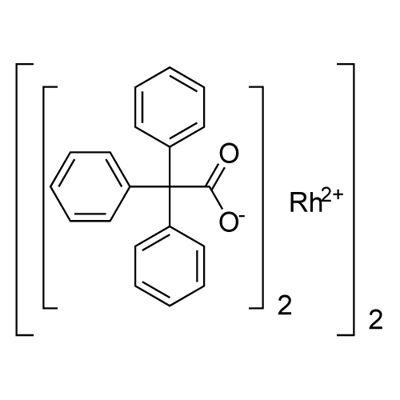

You may like
-
 Rhodium 99%View Details
Rhodium 99%View Details -
 Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
7440-16-6 -
 Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
7440-16-6 -
 Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
Rhodium, Rh 1000μg/mL CAS 7440-16-6View Details
7440-16-6 -
 Rhodium, 1% on alumina powder, Reduced CAS 7440-16-6View Details
Rhodium, 1% on alumina powder, Reduced CAS 7440-16-6View Details
7440-16-6 -
 Rhodium, 1% on alumina powder, Reduced CAS 7440-16-6View Details
Rhodium, 1% on alumina powder, Reduced CAS 7440-16-6View Details
7440-16-6 -
 Rhodium powder CAS 7440-16-6View Details
Rhodium powder CAS 7440-16-6View Details
7440-16-6 -
 Rhodium ICP standard CASView Details
Rhodium ICP standard CASView Details
