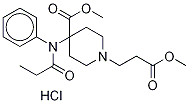
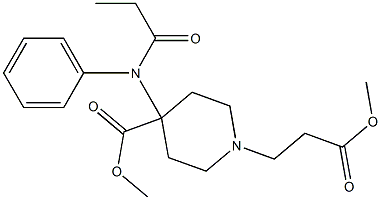
Remifentanil Hydrochloride
- CAS NO.:132539-07-2
- Empirical Formula: C20H28N2O5.ClH
- Molecular Weight: 412.911
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:37
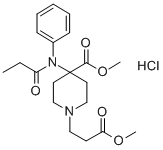
What is Remifentanil Hydrochloride?
Description
Ultiva was launched in Germany and the US for use in general anesthesia and immediate post-operative pain management. This compound can be prepared via the Michael addition of a 4,4-disubstituted piperidine to methyl acrylate. It behaves as a specific μ-opiod agonist that is about 30 times more potent than its chemical relative alfentanil. While its pharmacodynamic properties are similar to other μ agonists, remifentanil has unique pharmacokinetic properties. It has a rapid onset (1.6 min) and a rapid offset (5.4 min) independent of duration of administration. This has been attributed to its rapid catabolism by general esterases to the corresponding acrylic acid derivative. The metabolite has 0.01 to 0.0005 times the activity of remifentanil and is excreted via the kidneys (80% recovery).
Chemical properties
White Solid
Originator
Glaxo Wellcome (UK)
The Uses of Remifentanil Hydrochloride
Labelled synthetic mu-opioid agonist. This is a controlled substance (opiate)
brand name
Ultiva (Abbott).
Biochem/physiol Actions
Remifentanil hydrochloride is a mu opioid receptor agonist, anesthetic, and analgesic compound. Remifentanil was developed as an ultra-short-acting mu opioid receptor agonist with improved pharmacodynamic properties.
Pharmacokinetics
Remifentanil is much like alfentanil in its pharmacodynamic effects. It is a selective μ opioid agonist with 15 to 20 times greater potency than alfentanil. Remifentanil has an onset of action of 1 to 3 minutes when given intravenously. Its unique property is its rapid offset of action, which is independent of the duration of administration of the compound. Thus, it is very useful for titration of antinociceptive effect, followed by a rapid and predictable recovery time of 3 to 5 minutes. The short duration of action is a result of the ester group, which has been rationally designed into the substituent on the piperidine nitrogen. This ester group is rapidly hydrolyzed to the inactive carboxylic acid by serum and tissue esterases, making the drug's duration of action essentially independent of the liver or renal function of the patient. Remifentanil is used extensively for analgesia associated with general anesthesia procedures. It often is used in combination with injectable general anesthetic agents, such as midazolam or propofol.
Properties of Remifentanil Hydrochloride
| Melting point: | 195-197°C |
| Flash point: | 9°C |
| storage temp. | 2-8°C |
| solubility | PBS (pH 7.2): 10 mg/ml |
| form | A neat solid |
| CAS DataBase Reference | 132539-07-2 |
Safety information for Remifentanil Hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Remifentanil Hydrochloride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1