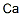radium
- CAS NO.:7440-14-4
- Empirical Formula: Ra
- Molecular Weight: 226
- MDL number: MFCD03700470
- EINECS: 231-122-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
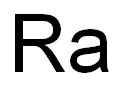
What is radium ?
Description
Radium has the symbol Ra and atomic number 88. Its
atomic weight is 226.0254 g/mol. Radium is an alkaline earth metal that is found in trace amounts in uranium
ores. Its most stable isotope, 226Ra, has a half-life of
1602 years and decays into radon gas.
The heaviest of the alkaline earth elements, radium is
intensely radioactive and resembles barium in its chemical
behavior. This metal is found in tiny quantities in the
uranium ore “Pitchblende”, and various other uranium
minerals. Radium preparations are remarkable for maintaining
themselves at a higher temperature than their
surroundings, and for their radiations, which are of
three kinds: alpha particles, beta particles and gamma
rays.
When freshly prepared, pure radium metal is almost pure white, but blackens when exposed to air (probably due to nitride formation). Radium is luminescent when struck by electromagnetic radiation of the proper wavelength (giving a faint blue color). It reacts violently with water to form radium hydroxide and is slightly more volatile than barium. The normal phase of radium is a solid. Since all the isotopes of radium are radioactive and short-lived on the geological time scale, any primeval radium would have disappeared long ago. Therefore, radium occurs naturally only as a disintegration product in the three natural radioactive decay series (Thorium, Uranium, and Actinium series). Radium-226 is a member of the uranium decay series. Its parent is Thorium-230 and its daughter Radon-222. Radium is a decay product of uranium and is therefore found in all uranium-bearing ores. (One ton of Pitchblende yields one seventh of a gram of radium). Radium was originally acquired from pitchblende ore from the Czech Republic. Carnotite (K2(UO2)2(VO4)2·3H2O) sands in Colorado provide some of the element, but richer ores are found in the Democratic Republic of Congo and the Great Lakes area of Canada. Radium can also be extracted from uranium processing waste.
Chemical properties
Brilliant-white solid. Luminescent, turns black on exposure to air. Soluble in water with evolution of hydrogen; forms water-soluble compounds. Decays by emis- sion of α-, β-, and γ-radiation. Bone-seeking when taken into the body.
Chemical properties
Radium (Ra) is a radioactive element, found naturally occurring in the environment. Ra is a silvery- white-metallic solid @ 25℃; it tarnishes black when exposed to air. It is an alkaline earth metal; there are 33 isotopes, all of them are unstable. Radium is commonly available as radium bromide (RaBr2 ) or radium chloride (RaCl2 ).
Physical properties
Radium is the last element in group 2 and is very similar to the other alkali earth metals,which makes it the largest and heaviest element in the group. It particularly resembles barium,which is just above it in group 2 of the periodic table. Radium is a bright white radioactiveluminescent alkali earth metal that turns black when exposed to air. Its melting point is700°C, its boiling point is 1,140°C, and its density is approximately 5.0 g/cm3.
Isotopes
There are no stable isotopes of radium. Radium has 25 known radioisotopes,ranging from Ra-206 to Ra-230. Their half-lives range from a fraction of a second tohundreds of years. Radium-226 was discovered by the Curies and has a half-life ofabout 1630 years. Ra-226 is the most abundant isotope, and thus, Ra-226 is used todetermine radium’s atomic mass.Various radium isotopes are derived through a series of radioactive decay processes. Forexample, Ra-223 is derived from the decay of actinium. Ra-228 and Ra-224 are the resultof the series of thorium decays, and Ra-226 is a result of the decay of the uranium series.
Isotopes
Radium (Ra) has no stable isotopes. A standard atomic mass cannot be given (but is usually given as 226.0 g/mol). The longest lived, and most common, isotope of radium is 226Ra that occurs in the disintegration chain of 238U (often referred to as the radiumseries). Radium (Ra) has 33 different known isotopes, four of which are found in nature, with 226Ra being the most common. 223Ra, 224Ra, 226Ra and 228Ra are all generated naturally in the decay of either Uranium (U) or Thorium (Th). 226Ra is a product of 238U decay, and is the longestlived isotope of radium with a half-life of 1602 years. The next longest is 228Ra, a product of 232Th breakdown, with a half-life of 5.75 years.
Origin of Name
Radium’s name is derived from the Latin word radius, which means “ray.
Occurrence
Radium is the 85th most abundant element found in the Earth’s crust. Radium is found inthe uranium ores pitchblende and chalcolite, which are both very radioactive. Radium metalexists to the extent of only one part to every three million parts of the uranium ore (pitchblende). Only about one gram of radium is found in every seven or eight tons of uraniumore. This scarcity seems to be the reason that only about five pounds of uranium are producedeach year in the entire world. Uranium ores are found in the states of Utah, New Mexico, andColorado in the United States and in Canada, the Czech Republic, Slovakia, Russia, Zaire,and France.
History
Curie in the pitchblende or uraninite of North Bohemia (Czech Republic), where it occurs. There is about 1 g of radium in 7 tons of pitchblende. The element was isolated in 1911 by Mme. Curie and Debierne by the electrolysis of a solution of pure radium chloride, employing a mercury cathode; on distillation in an atmosphere of hydrogen this amalgam yielded the pure metal. Originally, radium was obtained from the rich pitchblende ore found at Joachimsthal, Bohemia. The carnotite sands of Colorado furnish some radium, but richer ores are found in the Republic of Congo-Kinshasa and the Great Bear Lake region of Canada. Radium is present in all uranium minerals, and could be extracted, if desired, from the extensive wastes of uranium processing. Large uranium deposits are located in Ontario, New Mexico, Utah, Australia, and elsewhere. Radium is obtained commercially as the bromide or chloride; it is doubtful if any appreciable stock of the isolated element now exists. The pure metal is brilliant white when freshly prepared, but blackens on exposure to air, probably due to formation of the nitride. It exhibits luminescence, as do its salts; it decomposes in water and is somewhat more volatile than barium. It is a member of the alkaline-earth group of metals. Radium imparts a carmine red color to a flame. Radium emits alpha, beta, and gamma rays and when mixed with beryllium produce neutrons. One gram of 226Ra undergoes 3.7 × 1010 disintegrations per s. The curie (Ci) is defined as that amount of radioactivity which has the same disintegration rate as 1 g of 226Ra. Thirty-six isotopes are now known; radium 226, the common isotope, has a half-life of 1599 years. One gram of radium produces about 0.0001 mL (stp) of emanation, or radon gas, per day. This is pumped from the radium and sealed in minute tubes, which are used in the treatment of cancer and other diseases. One gram of radium yields about 4186 kJ per year. Radium is used in producing self-luminous paints, neutron sources, and in medicine for the treatment of cancer. Some of the more recently discovered radioisotopes, such as 60Co, are now being used in place of radium. Some of these sources are much more powerful, and others are safer to use. Radium loses about 1% of its activity in 25 years, being transformed into elements of lower atomic weight. Lead is a final product of disintegration. Stored radium should be ventilated to prevent build-up of radon. Inhalation, injection, or body exposure to radium can cause cancer and other body disorders. The maximum permissible burden in the total body for 226Ra is 7400 becquerel.
Characteristics
Radium is extremely radioactive. It glows in the dark with a faint bluish light. Radium’sradioisotopes undergo a series of four decay processes; each decay process ends with a stableisotope of lead. Radium-223 decays to Pb-207; radium-224 and radium-228decay to Pb-208;radium-226 decays to Pb-206; and radium-225 decays to Pb-209. During the decay processesthree types of radiation—alpha (α), beta (β), and gamma (γ)—are emitted.In addition to being radioactive, radium is extremely chemically reactive and forms manycompounds. These radium compounds are not only radioactive but also toxic and should behandled by experienced personnel.
History
Radium (Latin radius, ray) was discovered by Pierre
Curie, Marie Curie, and an assistant, G. Be′mont. This
occurred after Marie Curie had observed that the radioactivity
of pitchblende was four or five times greater
than that of the uranium it contained and was not fully
explained on the basis of radioactive polonium, which
she had just discovered in pitchblende residues originating
from North Bohemia, in the Czech Republic.
While studying pitchblende the Curies removed
uranium from it and found that the remaining material
was still radioactive. They then separated out a radioactive mixture consisting mostly of barium that
produced a brilliant green flame color and crimsoncarmine
spectral lines that had never been documented
before. The Curies announced their discovery to the
French Academy of Sciences on 26 December 1898.
In 1910, radium was isolated as a pure metal by Curie
and Debierne through the electrolysis of a pure radium
chloride solution by using a mercury cathode and
distilling it in an atmosphere of hydrogen gas. The separation
was followed by the increase in intensity of the
new lines in the ultraviolet spectrum and by a steady
increase in the apparent atomic weight of the material
until a value of 225.18 was obtained, remarkably close
to the accepted value of 226.03. By 1902, 0.1 g of pure
radium chloride was prepared by refining several tons
of pitchblende residues, and by 1910 Marie Curie and
Andre′-Louis Debierne had isolated the metal itself.
Radium was first industrially produced in the beginning
of the twentieth century by Birac, a subsidiary
company of UMHK in its Olen plant in Belgium. This
company offered to Marie Curie her first gram of
radium. Historically the decay products of radium
were known as radium A, B, C, etc.
The Uses of radium
Radium’s most important use is as a source of radiation in industry, medicine, and laboratories. The isotope radium-226, which is the most abundant of all the 25 isotopes and has ahalf-life of 1630 years, is the only useful form of the element. It is used in the medical treatment of malignant cancer growth. It kills cancer cells that have spread throughout the body.Other uses are to produce phosphorescence and fluorescence in organic compounds andfor scintillation screens on instruments used to detect radiation. Radium salts were used in thepast to paint the dials of luminous clock faces that glow in the dark.
The Uses of radium
Medical treatment for malignant growths, ind- ustrial radiography, source of neutrons and radon.
The Uses of radium
In 1909, the famous Rutherford experiment used radium as an alpha source to probe the atomic structure of gold. This experiment led to the Rutherford model of the atom and revolutionized the field of nuclear physics. Radium (usually in the form of RaCl2) was used in medicine to produce radon gas which in turn was used as a cancer treatment. For example, several radon sources were used in Canada in the 1920s and 1930s. The isotope 223Ra is currently under investigation for its use in cancer treatment of bone metastasis. Some of the few practical uses of radium are derived from its radioactive properties. More recently discovered radioisotopes, such as 60Co and 137Cs are replacing radium in even these limited uses because several of these isotopes are more powerful emitters, safer to handle, and available in more concentrated form.
Definition
Radioactive element of group IIA of the periodic table, atomic number 88, aw 226.0254, valence = 2. There are 14 radioactive isotopes but only radium-226 with half-life of 1620 years is usable. Discovered by the Curies in 1898.
Definition
A white radioactive luminescent metallic element of the alkaline- earth group. It has several short-lived radioisotopes and one long-lived isotope, radium-226 (half-life 1602 years). Radium is found in uranium ores, such as the oxides pitchblende and carnotite. It was formerly used in luminous paints and radiotherapy. Symbol: Ra; m.p. 700°C; b.p. 1140°C; r.d. 5 (approx. 20°C); p.n. 88; r.a.m. 226.0254 (226Ra).
Definition
radium: Symbol Ra. A radioactivemetallic element belonging togroup 2 (formerly IIA) of the periodictable; a.n. 88; r.a.m. 226.0254;r.d. ~5; m.p. 700°C; b.p. 1140°C. It occursin uranium ores (e.g. pitchblende).The most stable isotope isradium–226 (half-life 1602 years),which decays to radon. It is used as aradioactive source in research and, tosome extent, in radiotherapy. The elementwas isolated from pitchblendein 1898 by Marie and Pierre Curie.
Hazard
Highly toxic, emits ionizing radiation. Lead shielding should be used in storage and handling, adequate protective clothing and remote control devices are essential. Destructive to living tissue.
Hazard
Because radium energetically emits three types of radiation, it poses great danger to anyone handling it. In addition, it is toxic. If it is ingested in even small amounts, it replaces bone tissue, which can result in radiation sickness and death.
One of the decay products of radium is the gas radon, which can seep up through the Earth s crust into basements and slab level homes. Good ventilation assures that the radon does not accumulate to the extent that would be harmful.
At one time, women painted clock and watch dials with luminous radium paint that was a mixture of radium salts and zinc sulfide. They would place the small brushes between their lips and tongue to make the bristles more pointed, in order to paint fine lines with the radium paint. Over the years, they developed cancers that resulted in badly eaten-away and disfigured lips and jaws. Once the danger was known, luminous radium paint was banned for this use. Today, promethium (Pm-147), with a half-life of 2.4 years, is used for this purpose.
Safety Profile
A highly radiotoxic element. 1 g produces 3.7 x 1010 disintegrations per second. Inhalation, ingestion, or bodily exposure can lead to lung cancer, bone cancer, osteitis, skin damage, and blood dyscrasias. A common air contaminant. Radum replaces calcium in the bone structure and is a source of irradation to the blood-forming organs. The ingestion of luminous dial paint prepared from radium caused death in many of the early dal painters before the hazard was fully understood. The data on these workers have been the source of many of the radation precautions and the maximum permissible levels for internal emitters that are now accepted. 226Ra is the parent of radon and the precautions described under 222Rn should be followed. 22*Ra is a member of the thorium series. It was a common constituent of luminous paints, and, whde its low beta energy was not a hazard, its daughters in the series may have been a causative agent in the deaths of the radium dal painters following World War I. It is metabolized in the same way as any other radum isotope and it is a source of thorium. The precautions recommended under ZZORn should be followed. Highly dangerous; must be kept heavily shielded and stored away from possible dissemination by explosion, flood, etc. Radation Hazard: Natural isotope 223Ra (Actinium-X, Actinium Series), T1/2 = 11.4 days, decays to radoactive 219Rn by alphas of 5.5-5.7 MeV. Natural isotope 224Ra (Thorium-X, Thorium Series), T1/2 = 3.6 days, decays to radioactive 22ORn by alphas of 5.7 MeV. Natural isotope 226Ra (Uranium Series), T1/2 = 1600 years, decays to radoactive 222Rn by alphas of 4.8 MeV. Natural isotope 22*Ra (Mesothorium = 1 , Thorium Series), T1/2 = 6.7 years, decays to radoactive 22*Ac by betas of 0.05 MeV.
Potential Exposure
Radium is not available as a pure metal but is found in very small quantities in uranium and thorium ores. Uranium and thorium are found in small amounts in most rocks and soil; radium is formed when these elements break down in the environment. One ton of uranium ore yields only slightly more than 0.1 gm of radium. Radium is formed from the radioactive decay; and, as a by-product of refining these ores. Radium exists in several isotope forms. Two of the principal radium isotopes found in the environment are 226 Ra (radium-226) and 228 Ra (radium-228). Radium compounds, due to their geologically short half-life and intense radioactivity, are quite rare. A single gram of 226 Ra produces 10 24 mm of radon (Rn) a day. Radium’s- 226, radium’s most stable isotope has a half-life of about 1603 to 1620 years, and remains in the body for life. Radium, when used to produce radon gas, is used for treating various types of cancer; in radiography of metals; and combined with other metals, such as beryllium, as a neutron emitting source used in research and for cali- brating radiation instruments. Until the 1960s, radium was a component in self-luminous paints used for watch, com- pass, and aircraft instrument dials and other aircraft and military instrumentation. A less dangerous radioactive source, 60 Co (cobalt-60), replaced radium in luminous paint. The greatest health risk from radium comes from exposure to its radioactive decay product, radon (Rn). Radon is common in many soils and can collect in build- ings, including homes.
First aid
Unless you are dressed in appropriate protectivegear to prevent self-contaminating, do not provide medicalattention. Evacuate the victim from area of exposure to asafe area as soon as possible. To stop ongoing contamination, have the victim remove clothing, if possible, and placeclothing in a sealed garbage bag or container. Check thevictim’s breathing and pulse; start CPR, if necessary.Skin: If skin contamination has occurred, measure levels ofcontamination with a survey meter, record results, and begindecontamination by gentle washing with plenty of water(warm if possible) and nonabrasive and disinfecting soap,washing downwards towards extremities, not upwards. Drybody and cover the irritated skin with an emollient. If burnsare obvious, do not use ointments. Wrap victim in a clean,soft blanket. Seek immediate medical attention; evacuatethe victim to nearest emergency medical facility.Eyes: Check for and remove any contact lenses.Immediately flush eyes with cold water. Avoid the use ofan eye ointment. Seek immediate medical attention; evacuate the victim to nearest emergency medical facility.Inhalation: Allow the victim to rest in a well-ventilatedarea. If breathing is difficult, administer oxygen. Seekimmediate medical attention; evacuate the victim to nearestemergency medical facility.Ingestion: Do not induce vomiting. Loosen tight clothingsuch as a collar, tie, belt. Seek immediate medical attention;evacuate the victim to nearest emergency medical facility.
Carcinogenicity
The U.S. EPA has classified radium as a class A carcinogen, meaning that there are sufficient data to support a link between exposure to radium and the development of human cancer. The α-particle radiation associated with radium is recognized as the causative agent for sarcomas of the bone and carcinomas of the paranasal sinuses in humans. A study attempting to ascertain the dose–incidence relationship for induction of these tumors examined 1474 women employed in the U.S. radium dial painting industry before 1930. This population exhibited 61 known cases of bone sarcoma and 21 cases of carcinoma of the paranasal sinuses or the mastoid air cells. Of these individuals, the radium body burden was known for 759, among whom there were 38 cases of bone sarcoma and 17 head carcinomas.
Environmental Fate
Radium is released to the environment from a variety of sources, including milling and mining operations focused on phosphates and uranium, waste streams from factories used to produce products containing radium, or waste byproducts from production, such as phosphate fertilizers. The most important route of radium release into the environment is the combustion of coal, and released radium is estimated at 150 Ci per year (or 5.55×1012 Bq). Radium released into the atmosphere will remain in particulate form until wet or dry deposition. Radium adsorbs to soils, and is not expected to volatize from soils or water due to its ionic nature. In water, radium exists as Ra+2 and can interact with sediments and dissolve in water, and therefore generally adsorbs to sediments at the emission site and is not transported. Some isotopes of radium are very short lived, but radium-226 has a half-life of 1600 years, making long-range transport more likely, though generally only through an atmospheric route. Radium’s ionic nature makes bioaccumulation or biomagnification unlikely.
Storage
Store in a ventilated area to prevent accumulationof radon (Rn).
Shipping
UN3323(does not appear in the 49CFR hazard materials tables ) Radioactive material, Type C package, nonfissile or fissile excepted. UN2915 Radioactive mate- rial, Type A package nonspecial form, nonfissile or fissile- excepted, Hazard class: 7-Radioactive material; Labels: None. A1 and A2 values for Radium- 226 taken from y173.435 (see also Table A-1 in 10CFR71(Appendix A): A1 (SpecialForm * ) 0.3 TBq (8.11Ci); A2 (NormalForm) 0.02TBq (0.541Ci))
Toxicity evaluation
The radioactive properties of radium are the greatest concern and overwhelm all others. All radioactive materials may cause harm when decay particles are released; they disrupt many critical cell functions, includingDNA replication. Radioactive materials may also produce toxicity not related to their radioactive behavior. Like barium compounds, radium enters teeth and bones, altering growth and causing them to be weak and brittle.
Incompatibilities
Metallic radium is highly chemically reactive. It forms compounds that are very similar to barium compounds, making separation of the two elements difficult. On contact with water, radium forms flammable hydrogen gas.
Waste Disposal
Radioactive material consid- ered waste and must be retained in containers for disposi- tion by the authorizing institution. Drain disposal is prohibited. It is the responsibility of the operating institu- tion to arrange for the proper disposal of all forms of any radioisotopes. The use, storage, transportation, labeling, and disposal of radioactive material are regulated through the Nuclear Regulatory Commission (NRC) using 10 CFR (Code of Federal Regulations) as the regulatory basis and 49 CFR (Transportation).
Properties of radium
| Melting point: | 700° |
| Boiling point: | bp 1737° |
| Density | 5.5 |
| form | white metal |
| color | white metal; cubic |
| Water Solubility | evolves H2 in H2O [CRC10] |
| EPA Substance Registry System | Radium (7440-14-4) |
Safety information for radium
Computed Descriptors for radium
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8