Germanium
- CAS NO.:7440-56-4
- Empirical Formula: Ge
- Molecular Weight: 72.64
- MDL number: MFCD00085310
- EINECS: 231-164-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
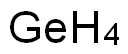
What is Germanium?
Description
Germanium is extracted from zinc ores in a very complicated process as it has aqueous properties similar to those of zinc. Once the germanium/zinc mixture has been sufficiently enriched with germanium, it is heated in HCl with Cl2 in order to allow the formation of germanium tetrachloride (GeCl2).
Chemical properties
Germanium is a grayish-white, lustrous, and brittle metalloid. The powder is grayish-black and odorless. It is never found free and occurs most commonly in ergyrodite and germanite. It is generally recovered as a by-product in zinc production, coal processing, or other sources.
Physical properties
Germanium has a gray shine with a metallic silvery-white luster. It is a brittle elementclassed as a semimetal or metalloid, meaning it is neither a metal such as iron or copper nora nonmetal, such as phosphorus, sulfur, or oxygen. Germanium has some properties likea metal and some like a nonmetal. It is a crystal in its pure state, somewhat like silicon. Itwill combine with oxygen to form germanium dioxide, which is similar to silicon dioxide(sand).
Germanium is not found in its free elemental state because it is much too reactive. For themost part, it is found combined with oxygen, either as germanium monoxide or as germaniumdioxide. Also, it is recovered from the ores of zinc, copper, and arsenic and the flue depositsof burning coal.
The crystal structure of germanium is similar to that of diamonds and silicon, and its semiconductingproperties are also similar to silicon.The melting point of germanium is 938.3°C, its boiling point is 2833°C, and its densityis 5.323 g/cm3.
Isotopes
There are a total of 38 isotopes of Germanium, five of which are stable. Thestable isotopes of germanium and their natural abundance are as follows: Ge-70 =20.37%, Ge-72 = 27.31%, Ge-73 = 7.76%, Ge-74 = 36.73%, and Ge-76 = 7.83%.Ge-76 is considered stable because it has such a long half-life (0.8×10+25 years)All theother 33 isotopes are radioactive and are produced artificially.
Origin of Name
Germanium’s name was derived from the Latin word Germania, meaning “Germany.”
Occurrence
Germanium, the 52nd most abundant element in the Earth’s crust, is widely distributed,but never found in its natural elemental state. It is always combined with other elements,particularly oxygen.
Germanium’s main minerals are germanite, argyrodite, renierite and canfieldite, all ofwhich are rare. Small amounts of germanium are found in zinc ore, as well as in copper andarsenic ores. It is known to concentrate in certain plants on Earth, particularly in coal: commercialquantities are collected from the soot in the stacks where coal is burned.
History
Germanium was predicted by Mendeleev in 1871 as ekasilicon, and discovered by Winkler in 1886. The metal is found in argyrodite, a sulfide of germanium and silver; in germanite, which contains 8% of the element; in zinc ores; in coal; and in other minerals. Germanium is frequently obtained commercially from flue dusts of smelters processing zinc ores, and has been recovered from the by-products of combustion of certain coals. Its presence in coal insures a large reserve of the element in the years to come. Germanium can be separated from other metals by fractional distillation of its volatile tetrachloride. The tetrachloride may then be hydrolyzed to give GeO2; the dioxide can be reduced with hydrogen to give the metal. Recently developed zone-refining techniques permit the production of germanium of ultra-high purity. The element is a gray-white metalloid, and in its pure state is crystalline and brittle, retaining its luster in air at room temperature. Germanium is a very important semiconductor material. Zone-refining techniques have led to production of crystalline germanium for semiconductor use with an impurity of only one part in 1010. Doped with arsenic, gallium, or other elements, it is used as a transistor element in thousands of electronic applications. Its application in fiber optics and infrared optical systems now provides the largest use for germanium. Germanium is also finding many other applications including use as an alloying agent, as a phosphor in fluorescent lamps, and as a catalyst. Germanium and germanium oxide are transparent to the infrared and are used in infrared spectrometers and other optical equipment, including extremely sensitive infrared detectors. Germanium oxide’s high index of refraction and dispersion make it useful as a component of glasses used in wide-angle camera lenses and microscope objectives. The field of organogermanium chemistry is becoming increasingly important. Certain germanium compounds have a low mammalian toxicity, but a marked activity against certain bacteria, which makes them of interest as chemotherapeutic agents. The cost of germanium is about $10/g (99.999% purity). Thirty isotopes and isomers are known, five of which occur naturally.
Characteristics
Once germanium is recovered and formed into blocks, it is further refined by the manufacturerof semiconductors. It is melted, and the small amounts of impurities such as arsenic, gallium,or antimony, are added. They act as either electron donors or acceptors that are infused(doped) into the mix. Then small amounts of the molten material are removed and used togrow crystals of germanium that are formed into semiconducting transistors on a germaniumchip. The device can now carry variable amounts of electricity because it can act as both aninsulator and a conductor of electrons, which is the basis of modern computers.
The Uses of Germanium
In electronics: manufacture of rectifying devices (germanium diodes), transistors, in red-fluorescing phosphors; in dental alloys; in the production of glass capable of transmitting infrared radiation. Review of uses: Aldington, Cumming, Endeavour 14, 200-204 (1955); New Uses for Germanium, F. I. Metz, Ed. (Midwest Research Institute, 1974) 120 pp.
The Uses of Germanium
By far, the most common use for germanium is in the semiconductor and electronicsindustries. As a semiconductor, germanium can be used to make transistors, diodes, andnumerous types of computer chips. It was the first element that could be designed to act asdifferent types of semiconductors for a variety of applications just by adding variable amountsof impurities (doping) to the germanium crystals.
Germanium is also used as a brazing alloy, for producing infrared transmitting glass andother types of lenses, and for producing synthetic garnets (semiprecious gemstones) that havespecial magnetic properties.
The Uses of Germanium
The product can serve as one of the precursors for the formation of highly porous ZrO2:Tb3+ nanophosphor with excellent tunable photoluminescence and photocatalytic activities.
What are the applications of Application
Germanium is a chemical element which naturally reacts and forms complexes with oxygen in nature
Definition
A hard brittle gray metalloid element belonging to group 14 (formerly IVA) of the periodic table. It is found in sulfide ores such as argyrodite (4Ag2S·GeS2) and in zinc ores and coal. Most germanium is recovered during zinc or copper refining as a by-product. Germanium was extensively used in early semiconductor devices but has now been largely superseded by silicon. It is used as an alloying agent, catalyst, phosphor, and in infrared equipment. Symbol: Ge; m.p. 937.45°C; b.p. 2830°C; r.d. 5.323 (20°C); p.n. 32; r.a.m. 72.61.
Definition
germanium: Symbol Ge. A lustroushard metalloid element belonging togroup 14 (formerly IVB) of the periodictable; a.n. 32; r.a.m. 72.59; r.d.5.36; m.p. 937°C; b.p. 2830°C. It isfound in zinc sulphide and in certainother sulphide ores, and is mainlyobtained as a by-product of zincsmelting. It is also present in somecoal (up to 1.6%). Small amounts areused in specialized alloys but themain use depends on its semiconductorproperties. Chemically, it formscompounds in the +2 and +4 oxidationstates, the germanium(IV) compoundsbeing the more stable. Theelement also forms a large numberof organometallic compounds. Predictedin 1871 by Mendeleev (ekasilicon),it was discovered by Winklerin 1886.
Production Methods
The concentration of germanium in the earth’s crust is approximately 7 ppm. Germanium is not found in the free state, but in combination with other elements as a mineral, such as in the mineral argyrodite (Ag8GeS6, 5–7% Ge) and germanite (7CuS–FeS2–GeS2, 8.7% Ge). Enargite, a Cu–As sulfide, is found in the western United States and contains as much as 0.03% Ge; however, none of these minerals are utilized for recovery of germanium because of the small quantities available. The principal domestic source of germanium is from the residues of cadmium derived from zinc ores. Commercial recovery of germanium has been chiefly from zinc and Zn–Cu–Pb ores, germanite, and flue dusts from coals. Some silver and tin ores contain germanium, as do many types of coal. Oak and beech humus in one locality in Germany reportedly contain 70 ppm germanium.
Hazard
Many of the chemicals used in the semiconductor industries are highly toxic. For example,germanium-halogen compounds are extremely toxic, both as a powder and in a gaseous state.Precautions should be taken when working with germanium as with similar metalloids fromgroup 14 (IVA).
Flammability and Explosibility
Not classified
Industrial uses
A rare elemental metal, germanium (Ge) has agrayish white crystalline appearance and hasgreat hardness: 6.25 Mohs. Its specific gravityis 5.35, and melting point is 937°C. It is resistantto acids and alkalies. It has metallic-appearingcrystals with diamond structure, givesgreater hardness and strength to aluminum andmagnesium alloys, and as little as 0.35% in tinwill double the hardness. It is not used commonlyin alloys, however, because of its rarityand great cost. It is used chiefly as metal inrectifiers and transistors. An Au–Ge alloy, withabout 12% germanium, has a melting point of359°C and has been used for soldering jewelry.
Germanium is obtained as a by-productfrom flue dust of the zinc industry, or it can beobtained by reduction of its oxide from the ores,and is marketed in small irregular lumps. Germaniumcrystals are grown in rods up to 3.49cm in diameter for use in making transistorwafers. High-purity crystals are used for both P and Nsemiconductors. They are easier topurify and have a lower melting point than othersemiconductors, specifically silicon.
Industrial uses
Germanium lenses and filters have been usedin instruments that operate in the infraredregion of the spectrum. Windows and lenses ofgermanium are vital components of some laserand infrared guidance or detection systems.Glasses prepared with germanium dioxide havea higher refractivity and dispersion than docomparable silicate glasses and may be used inwide-angle camera lenses and microscopes.
Potential Exposure
Because of its semiconductor proper ties, germanium is widely used in the electronic industry in rectifiers, diodes, and transistors. It is alloyed with alumi num, aluminum magnesium, antimony, bronze, and tin to increase strength, hardness, or corrosion resistance. In the process of alloying germanium and arsenic, arsine may be released; stibine is released from the alloying of germanium and antimony. Germanium is also used in the manufacture of optical glass for infrared applications; red-fluorescing phosphors; and cathodes for electronic valves; and in elec troplating; in the hydrogenation of coal; and as a catalyst, particularly at low temperatures. Certain compounds are used medically. Industrial exposures to the dust and fumes of the metal or oxide generally occur during separation and purification of germanium, welding, multiple-zone melting operations, or cutting and grinding of crystals. Germanium tetrahydride (germanium hydride, germane, and monoger mane) and other hydrides are produced by the action of a reducing acid on a germanium alloy.
Environmental Fate
Metals are recalcitrant to degradation; therefore, no biodegradation studies have been performed on germanium compounds. Naturally occurring germanium exists in mineral ores; therefore, the levels of free germanium are expected to be low and of low concern for bioaccumulation in aquatic and terrestrial species, due to negligible exposures.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required.
Purification Methods
Copper contamination on the surface and in the bulk of single crystals of Ge can be removed by immersion in molten alkali cyanide under N2. The Ge is placed in dry K and/or Na cyanide powder in a graphite holder in a quartz or porcelain boat. The boat is then inserted into a heated furnace which, after a suitable time, is left to cool to room temperature. At 750o, a 1mm thickness of metal requires about 1minute, whereas 0.5cm needs about half hour. The boat is removed from the furnace, and the solid samples are taken out with plastic-coated tweezers, carefully rinsed in hot water and dried in air [Wang J Phys Chem 60 45 1956, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 712 1963]. Care with the use of cyanide.
Incompatibilities
A strong reducing agent and flammable solid. Finely divided metal is incompatible with oxidizing and nonoxidizing acids, ammonia, bromine, oxidizers, aqua regia, sulfuric acid, carbonates, halogens, and nitrates. Explosive reaction or ignition with potassium chlorate, potassium nitrate, chlorine, bromine, oxygen, and potas sium hydroxide in the presence of heat. Violent reaction with strong acids: aqua regia, nitric, and sulfuric. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions.
Waste Disposal
Recovery and return to sup pliers for reprocessing is preferable.
Properties of Germanium
| Melting point: | 937 °C (lit.) |
| Boiling point: | 2830 °C (lit.) |
| Density | 5.35 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Flammables area |
| solubility | insoluble in H2O, dilute acid solutions, alkaline solutions |
| form | powder |
| color | Silver |
| Specific Gravity | 5.35 |
| Resistivity | 53000 μΩ-cm, 20°C |
| Water Solubility | insoluble H2O, HCl, dilute alkali hydroxides; attacked by aqua regia [MER06] |
| Crystal Structure | Cubic, Diamond Structure - Space Group Fd3m |
| Merck | 13,4419 |
| Exposure limits | ACGIH: TWA 0.5 ppm(2.5 mg/m3); Ceiling 2 ppm (Skin) OSHA: TWA 3 ppm NIOSH: IDLH 30 ppm(250 mg/m3); TWA 3 ppm(2.5 mg/m3); Ceiling 6 ppm(5 mg/m3) |
| Stability: | Stable. Slightly soluble in strong acids. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7440-56-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Germanium(7440-56-4) |
| EPA Substance Registry System | Germanium (7440-56-4) |
Safety information for Germanium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H361:Reproductive toxicity H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Germanium
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
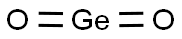
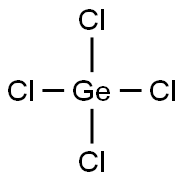
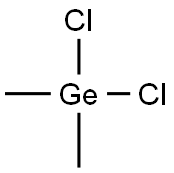
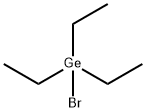

You may like
-
 Germanium CAS 7440-56-4View Details
Germanium CAS 7440-56-4View Details
7440-56-4 -
 Germanium CAS 7440-56-4View Details
Germanium CAS 7440-56-4View Details
7440-56-4 -
 Germanium CAS 7440-56-4View Details
Germanium CAS 7440-56-4View Details
7440-56-4 -
 Germanium CAS 7440-56-4View Details
Germanium CAS 7440-56-4View Details
7440-56-4 -
 Germanium CAS 7440-56-4View Details
Germanium CAS 7440-56-4View Details
7440-56-4 -
 Germanium rod, 6.35mm (0.25 in.) dia. CAS 7440-56-4View Details
Germanium rod, 6.35mm (0.25 in.) dia. CAS 7440-56-4View Details
7440-56-4 -
 Germanium optical (mono crystalline) windows, 100 mm dia, 20 mm thick CAS 7440-56-4View Details
Germanium optical (mono crystalline) windows, 100 mm dia, 20 mm thick CAS 7440-56-4View Details
7440-56-4 -
 Germanium pieces, ≤10 mm, 99.9% CAS 7440-56-4View Details
Germanium pieces, ≤10 mm, 99.9% CAS 7440-56-4View Details
7440-56-4
