rac 4-Hydroxy Ephedrine Hydrochloride
- CAS NO.:365-26-4
- Empirical Formula: C10H15NO2
- Molecular Weight: 181.23
- EINECS: 206-672-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-23 08:43:30
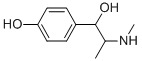
What is rac 4-Hydroxy Ephedrine Hydrochloride?
Originator
Carnigen, Aventis Pharma Deutschland
Indications
Used in combination with Normethadone as an antitussive .
Background
Oxilofrine is used in combination with Normethadone as an antitussive. It is currently marketed in Canada by Valeant under the tradename Cophylac.
Manufacturing Process
The p-benzyloxypropiophenone used for the transformation is prepared by adding to a solution of 6.9 g of sodium in 230 ml of absolute alcohol 45.0 g of p-hydroxypropiophenone and 54.0 g of benzyl bromide, and boiling for 1 h in a reflux apparatus. The excess of alcohol is then distilled and the residue is extracted with ether and water. After drying it the ethereal solution is evaporated and the residue is recrystallized from alcohol of 95% strength. The yield amounts to 60.0 g. The p-benzyloxypropiophenone melts at 100°-101°C.
55.0 g of p-benzyloxypropiophenone are dissolved in 250 ml of methylene chloride and brominated with 38.0 g of bromine. As soon as all of the bromine has been introduced drop by drop, the methylene chloride solution is washed with caustic soda solution and water. The solvent is eliminated in a vacuum and the residue is dissolved in petroleum ether. The crystalline mass which soon separates is filtered by suction and washed with petroleum ether. When recrystallized from hexahydrobenzene the p-benzyloxybromopropiophenone melts at 80°C.
40.0 g of p-benzyloxybromopropiophenone are then transformed in an alcoholic solution with 30.0 g of methylbenzylamine. After the whole has been allowed to stand for 1 day, the excess of alcohol is distilled in a vacuum and the residue is dissolved with ether. By washing with water, the benzylmethylamine hydrobrornide then formed is eliminated and the ether is removed by evaporation. The ether residue soon begins to crystallize and the p-benzyloxymethylbenzylaminopropriophenone is obtained with a good yield; when recrystallized from petroleum ether of low boiling point it melts at 58°60°C.
15.0 g of p-benzyloxymethylbenzylaminopropriophenone are dissolved in alcohol and the solution is hydrogenated with palladium and hydrogen. As soon as the required quantity of hydrogen has been absorbed the palladium is filtered by suction and the alcohol is evaporated in a vacuum The residue is recrystallized and the p-hydroxyphenylmethylaminopropanol is obtained.
Therapeutic Function
Sympathomimetic, Analeptic
Pharmacokinetics
Oxilofrine is a sympathomimetic which increases adrenergic activity .
Metabolism
Not Available
Properties of rac 4-Hydroxy Ephedrine Hydrochloride
| Melting point: | 152-154° |
| Boiling point: | 314.35°C (rough estimate) |
| Density | 1.0918 (rough estimate) |
| refractive index | 1.5464 (estimate) |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
| pka | 9.97±0.26(Predicted) |
| InChI | InChI=1S/C10H15NO2/c1-7(11-2)10(13)8-3-5-9(12)6-4-8/h3-7,10-13H,1-2H3 |
Safety information for rac 4-Hydroxy Ephedrine Hydrochloride
Computed Descriptors for rac 4-Hydroxy Ephedrine Hydrochloride
| InChIKey | OXFGTKPPFSCSMA-UHFFFAOYSA-N |
| SMILES | C1(=CC=C(O)C=C1)C(O)C(C)NC |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![4-[1-HYDROXY-2-([1-METHYLETHYL]AMINO)BUTYL]-1,2-BENZENEDIOL METHANESULFONATE](https://img.chemicalbook.in/CAS/GIF/7279-75-6.gif)
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
