(R)-(+)-2-Methyl-2-propanesulfinamide
- CAS NO.:196929-78-9
- Empirical Formula: C4H11NOS
- Molecular Weight: 121.2
- MDL number: MFCD05861479
- EINECS: 676-338-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 17:11:28
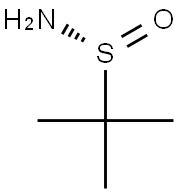
What is (R)-(+)-2-Methyl-2-propanesulfinamide?
Chemical properties
(R)-(+)-2-Methyl-2-propanesulfinamide is white to light yellow crystal powde
The Uses of (R)-(+)-2-Methyl-2-propanesulfinamide
(R)-(+)-2-Methyl-2-propanesulfinamide is a chiral ligand, which is used in pharmaceutical compositions. Further, it is used in the preparation of beta-chloro sulfinamides in the synthesis of chiral azridines. It is involved in the preparation of organocatalyst for enantioselective reduction of imines. It serves as a reagent for synthesizing chiral amines. In addition to this, it is converted into P,N-sulfinyl imine ligands through condensation with aldehydes and ketones which undergoes iridium-catalyzed asymmetric hydrogenation of olefins.
The Uses of (R)-(+)-2-Methyl-2-propanesulfinamide
Chiral ligand used in pharmaceutical compositions
The Uses of (R)-(+)-2-Methyl-2-propanesulfinamide
suzuki reaction, useful reagent for synthesizing chiral amines.
What are the applications of Application
(R)-tert-Butylsulfinamide is (R)-tert-Butylsulfinamide is a chiral ligand used in pharmaceutical compositions.
Preparation
Acetic acid (70 g), R-tert-butylsulfinylhydrazine (42 g), zinc powder (60.5 g) and dichloromethane (150 mL) were added to the reaction flask. The temperature was slowly heated to 35-42 °C . After 16 hours, the filtrate was poured into 70 mL water. Dichloromethane (75 g×5) was added for extraction. Collected the organic phase, added 48% NaOH to adjust ρΗ (7-8). Then added NaCl, the layers were separated and the organic phase was washed with 15 g saturated aqueous solution of sodium chloride. The solution was dried over magnesium sulfate. After filterED, filtrate was concentrated under reduced pressure at 25-30 °C until no slipping. N-heptane was replaced, 28 g mixed solvent of N-heptane and toluene (N-heptane: toluene = 6:1) were added to perform beating at low temperature, filtered to obtain (R)-(+)-2-Methyl-2-propanesulfinamide. Yield=83%
References
[1] Qian X, et al. A stereoselective synthesis of (S)-2-(((3-fluoro-4-methylphenoxy)carbonyl)(1-(4-((5-methyl-2-phenyloxazol-4-yl)methoxy)phenyl)ethyl)amino)acetic acid, a highly potent PPAR α/γ dual agonist. Tetrahedron, 2015; 71: 9408-9414.
Properties of (R)-(+)-2-Methyl-2-propanesulfinamide
| Melting point: | 103-107 °C(lit.) |
| Boiling point: | 220.0±23.0 °C(Predicted) |
| alpha | 4 º (c=1, CHCl3 + amylenes) |
| Density | 0.903 g/mL at 25 °C |
| refractive index | 4 ° (C=1, CHCl3) |
| Flash point: | -17℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in chloroform, methanol, tetrahydrofuran, dichloromethane, dimethyl sulfoxide and most organic solvents. |
| form | Powder |
| pka | 10.11±0.50(Predicted) |
| color | White, light pink, light yellow to brown |
| optical activity | [α]22/D +1.0°, c = 0.5% in chloroform |
| Stability: | store cold |
| CAS DataBase Reference | 196929-78-9(CAS DataBase Reference) |
Safety information for (R)-(+)-2-Methyl-2-propanesulfinamide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for (R)-(+)-2-Methyl-2-propanesulfinamide
| InChIKey | CESUXLKAADQNTB-SSDOTTSWSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 196929-78-9 (R)-(-)-2-Methyl-2-Propane sulfonamide 98%View Details
196929-78-9 (R)-(-)-2-Methyl-2-Propane sulfonamide 98%View Details
196929-78-9 -
 196929-78-9 99%View Details
196929-78-9 99%View Details
196929-78-9 -
 (R)-(+)-2-Methyl-2-propanesulfinamide, 98% CAS 196929-78-9View Details
(R)-(+)-2-Methyl-2-propanesulfinamide, 98% CAS 196929-78-9View Details
196929-78-9 -
 (R)-(+)-tert-Butylsulfinamide CAS 196929-78-9View Details
(R)-(+)-tert-Butylsulfinamide CAS 196929-78-9View Details
196929-78-9 -
 (R)-2-Methyl-2-propanesulfinamide 196929-78-9 98%View Details
(R)-2-Methyl-2-propanesulfinamide 196929-78-9 98%View Details
196929-78-9 -
 Chiral Sulfomide 99%View Details
Chiral Sulfomide 99%View Details
196929-78-9 -
 R)-(+)-2-methyl-2-propanesulfinamide CAS 196929-78-9View Details
R)-(+)-2-methyl-2-propanesulfinamide CAS 196929-78-9View Details
196929-78-9 -
 (R)-(+)-2-Methyl-2-propanesulfinamide CAS 196929-78-9View Details
(R)-(+)-2-Methyl-2-propanesulfinamide CAS 196929-78-9View Details
196929-78-9