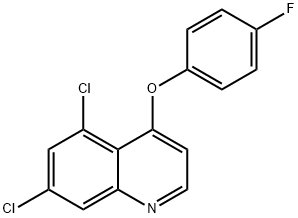
QUINOXYFEN
Synonym(s):(5,7-Dichloro-4-quinolyl) (4-fluorophenyl) ether
- CAS NO.:124495-18-7
- Empirical Formula: C15H8Cl2FNO
- Molecular Weight: 308.13
- MDL number: MFCD03265638
- EINECS: 602-997-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is QUINOXYFEN?
Chemical properties
Off-White Solid
The Uses of QUINOXYFEN
Quinoxyfen is under development for the control of powdery mildew in cereals and grapes.
The Uses of QUINOXYFEN
Agricultural fungicide.
What are the applications of Application
Quinoxyfen is a protectant fungicide used to control powdery mildew
Definition
ChEBI: A member of the class of quinolines carrying two chloro substituents at positions 5 and 7 together with a 4-fluorophenoxy substituent at position 4. A fungicide used mainly to control powdery mildew in cereals.
Environmental Fate
The stability of quinoxyfen in the soil has been shown
to vary depending on soil type and source. DT50 values
obtained in the field varied from 5 to 454 days for a
range of soil types. The strong adsorptive properties of
quinoxyfen reduce its soil dissipation rate, but result in
no leaching potential of this fungicide into waterways or
groundwater. The primary metabolite formed in the soil is
3-hydroxyquinoxyfen . A secondary soil metabolite
is 5,7-dichloro-4-hydroxyquinoline (DCHQ). DCHQ was
also found not to leach, even in sandy soils. Under acidic
aqueous conditions, DCHQ was the primary metabolite
found, and this was produced in greater quantities at
acidic pHs. An additional metabolite was isolated from
both water and the sediment in an aqueous clay loam
system. Although not positively identified, it is suspected
to be 6-hydroxyquinoxyfen.
A similar hydrolysis profile is observed in water
as in soil (3). The primary product produced under
acidic conditions in the absence of light was DCHQ.
However, in the presence of light, photolysis was greatly
increased and dose dependent on the amount of sunlight
received. The primary photolysis product was 2-chloro-10-
fluoro[1]benzopyrano[2,3,4-de]quinoline (CFBPQ).
Metabolic pathway
Quinoxyfen is a novel fungicide for the control of powdery mildew in
cereals. Its mode of action is unknown but it appears to differ from those
of current fungicides and thus may be novel (Longhurst et al., 1996).
Quinoxyfen is tightly bound to soil components and is somewhat persistent
in this medium but in aqueous solution it is subject to rapid
photodecomposition. Photodegradation is therefore likely to be an important
process in its immediate removal from the environment. Plant
metabolites have not been reported but unchanged quinoxyfen has been
confirmed as the major residue.
The compound is rapidly metabolised and eliminated following ingestion
by rats and goats. Metabolism involves mainly hydroxylation of the
intact quinoxyfen, cleavage at the ether bond and conjugation of the
resulting metabolites. The information presented below was obtained
from two sources (DowElanco, 1996; Reeves et al., 1996).
Toxicity evaluation
The specificity of quinoxyfen to fungi may account for its relatively safe toxicological profile. Quinoxyfen has no demonstrable effect in any genotoxicity tests, and the rat oral LD50 is >5000 mg/kg. Quinoxyfen was not shown to cause skin irritation, but studies with rabbit indicated it might cause mild eye irritation and it has the potential to cause skin sensitization, as shown in guinea pigs with repeated exposure. A NOEL of 20 mg/kg bw/day was established through 1-year and 2-year rat chronic feeding studies. The aquatic precautions with quinoxyfen may be due to its high logP. The rainbow trout LD50 is 0.27 mg/L; however, this is well above its water solubility of 0.116 mg/L. In addition, the Daphnia 48h EC50 is 0.08 mg/kg and the Selenastrum capricornutum 72 h EbC50 is 0.03 mg/kg. With the exception of some aquatic species, quinoxyfen has a very desirable toxicological profile toward nontarget species in the environment. Its toxicity toward birds, honeybees, and earthworms is low.
Degradation
Quinoxyfen is stable in the dark at 25 °C and it is stable in aqueous solution at pH 7 and 9. Its DT50 at pH 4 was 16 days (40°C) but it was degraded more rapidly than this in light. Aqueous photolysis of dilute solutions occurs with DT50 values of 1.7 and 22.8 hours in June and December (Europe), respectively. The main degradation product was 2-chloro-10-fluoro[1]benzopyrano[2,3,4-de]quinoline (2, up to 30%); a second product (up to 11%) was probably 5,7-dichloro-4-hydroxyquinoline (3) (see Scheme 1).
Properties of QUINOXYFEN
| Melting point: | 105-106° |
| Boiling point: | 423℃ |
| Density | 1.430 |
| vapor pressure | 1.2 x 10-5 Pa (20 °C) |
| Flash point: | >100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 2.87±0.50(Predicted) |
| form | neat |
| Water Solubility | 0.12 mg l-1 (20 °C) |
| color | White to Light yellow |
| Merck | 14,8079 |
| EPA Substance Registry System | Quinoxyfen (124495-18-7) |
Safety information for QUINOXYFEN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for QUINOXYFEN
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Quinoxyfen CAS 124495-18-7View Details
Quinoxyfen CAS 124495-18-7View Details
124495-18-7 -
 Quinoxyfen CAS 124495-18-7View Details
Quinoxyfen CAS 124495-18-7View Details
124495-18-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1