Pyriproxyfen
Synonym(s):2-[1-Methyl-2-(4-phenoxyphenoxy)ethoxy]pyridine
- CAS NO.:95737-68-1
- Empirical Formula: C20H19NO3
- Molecular Weight: 321.37
- MDL number: MFCD01691614
- EINECS: 429-800-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02

What is Pyriproxyfen?
Description
Pyriproxyfen is a pyridine compound and, in common with fenoxycarb, is a juvenile hormone mimic whose structure is unrelated to natural juvenile hormone. It is an insect growth regulator. Fleas absorb pyriproxyfen either by direct contact or by ingesting blood from a treated animal.
Description
Pyriproxyfen, like last week’s molecule, spinosyn A, is an insecticide. It is marketed in the United States under the trade names Distance and Nylar. Commonly used to combat fire ants, it is applied to the ground in a bait. Worker ants carry it to the colony, where it kills all of the ants, including the queen.
Description
Pyriproxyfen is a pyridine insecticide that mimics juvenile growth hormone, which prevents larvae from developing into reproduction-capable adults. The LD50 of pyriproxyfen in rats is >5,000 mg/kg, >1,300 mg/cubic meter/4 hours, and >2,000 mg/kg through oral, inhalation, or percutaneous dosing, respectively. It is used as a larvicide in the drinking water of 11 municipalities in Brazil and rumors suggested it may be correlated with the increase in cases of microcephaly in Brazil. The acceptable daily intake determined by the World Health Organization is 0.3 mg/L. The prevalence of microcephaly in Brazil is not higher in municipalities that use pyriproxyfen in the water supply, compared with municipalities that use the larvicide Bti. In addition, in zebrafish, even very high doses (0.1 μg/ml, compared with 0.01 μg/ml used in practice for pest control) pyriproxyfen does not induce microcephaly or other brain malformations. Formulations containing pyriproxyfen are used for flea control in dogs and as an insecticide for ants.
Chemical properties
Gray to white crystalline solid or powder. Also described as a pale yellow, waxy solid or liquid. Commercial product is available as an emulsifiable concen- trate or wettable powders.
The Uses of Pyriproxyfen
Pyriproxyfen is a pyridine-based pesticide used against a variety of arthropoda, in particular to protect cotton crops against whitefly.
The Uses of Pyriproxyfen
Insecticide.
The Uses of Pyriproxyfen
Pyriproxyfen is used for control of public health pests (flies, beetles, midges, mosquitoes) by application to breeding sites.
What are the applications of Application
Pyriproxyfen is a larvicidal agent that mimics juvenile insect hormone
Definition
ChEBI: An aromatic ether that consists of propylene glycol having a 2-pyridyl group at the O-1 position and a 4-phenoxyphenyl group at the O-3 position.
Agricultural Uses
Insect growth regulator, Insecticide, Veterinary medicine: Pyriproxyfen is found in a number of household products as sprays, powders, baits, mists and shampoos for the control of fleas, ticks, mites and flying insects on pets, in the air, and in carpets and rugs. It’s a larvicidal agent that mimics juvenile insect hormone.
Trade name
ARCHER®; DALAR®; DISTANCE®; ESTEEM®; NYLAR®; S-9318®; S 31183®; SUMILARV® Pyriproxyfen
Potential Exposure
Pyriproxyfen is an unclassified insect growth regulator, insecticide, veterinary medicine found in a number of household products as sprays, powders, baits, mists and shampoos for the control of fleas, ticks, mites, and flying insects on pets, in the air, and in carpets and rugs. It’s a l larvicidal agent that mimics juvenile insect hormone.
Metabolic pathway
Pyriproxyfen is not persistent in soils and sediments and is rapidly metabolised by a variety of organisms. In rats and mice, the major metabolic pathways were hydroxylation of the aromatic ring, hydroxylation at the 5-position of the pyridyl ring, loss of the aromatic ring, cleavage of ether linkages and conjugation of the resultant phenols with glucuronic or sulfuric acid. The general pathways in soils, sediments and a variety of organisms involve fission of ether linkages and hydroxylation of the phenoxyphenol group.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.
Degradation
[14C]Pyriproxyfen was dissolved in distilled water or sterile river water at a concentration of 0.2 mg kg-1 and exposed to natural sunlight from November to December at 40°N latitude. The DT50 values were 17.5 and 21 days in distilled water and sterile river water respectively. Photodegradation involved cleavage at the three ether linkages and the major products were carbon dioxide (11-29% of the applied radioactivity) and a product formed by loss of the phenoxyphenyl group (9) (16-30%). When [14C]pyriproxyfen was exposed to sunlight for 8 weeks on sandy loam and silty loam soils, the DT50 values were 11 and 13 weeks respectively. Photodegradation involved cleavage at the three ether linkages to give products 4,6 and 10 but the major product was carbon dioxide, representing 9.5-13% of the applied radioactivity. Bound radioactivity accounted for 11-26% of the applied radioactivity at week 8 (Miyamoto et al., 1993). Degradation products are shown in Scheme 1.
Incompatibilities
This material is combustible. Dust may form an explosive mixture in air. Incompatible with oxidi- zers (chlorates, nitrates, peroxides, permanganates, perchlo- rates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is the responsibility of chemical waste generators to determine the toxicity and physical properties and of a discarded chemical and to properly identify its classification and certification as a hazardous waste and to determine the disposal method. United States Environmental Protection Agency guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult and follow all regional, national, state and local hazardous waste laws to ensure complete and accurate classification and disposal methods. Follow recommendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Pyriproxyfen
| Melting point: | 45-47°C |
| Boiling point: | 230-250 °C(Press: 0.2 Torr) |
| Density | 1.32 |
| vapor pressure | 2.9 x l0-4 Pa (20 °C) |
| refractive index | nD20.5 1.5823 |
| storage temp. | 0-6°C |
| solubility | DMF: 30 mg/ml; DMF:PBS(pH7.2) (1:2): 0.33 mg/ml; DMSO: 25 mg/ml; Ethanol: 15 mg/ml |
| pka | 3.2 (base, est.) |
| Water Solubility | 0.4 mg l-1 (25 °C) |
| form | neat |
| form | Solid |
| color | White to off-white |
| InChI | InChI=1S/C20H19NO3/c1-16(23-20-9-5-6-14-21-20)15-22-17-10-12-19(13-11-17)24-18-7-3-2-4-8-18/h2-14,16H,15H2,1H3 |
| CAS DataBase Reference | 95737-68-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyriproxyfen(95737-68-1) |
| EPA Substance Registry System | Pyriproxyfen (95737-68-1) |
Safety information for Pyriproxyfen
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Pyriproxyfen
| InChIKey | NHDHVHZZCFYRSB-UHFFFAOYSA-N |
| SMILES | C1(OC(C)COC2=CC=C(OC3=CC=CC=C3)C=C2)=NC=CC=C1 |
Pyriproxyfen manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

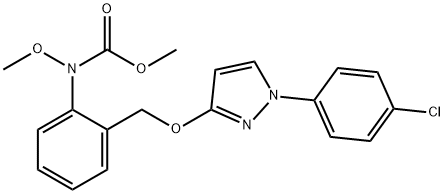
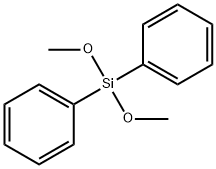
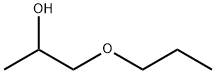
You may like
-
 95737-68-1 Pyriproxyfen 99%View Details
95737-68-1 Pyriproxyfen 99%View Details
95737-68-1 -
 95737-68-1 99%View Details
95737-68-1 99%View Details
95737-68-1 -
 Pyriproxyfen 97% CAS 95737-68-1View Details
Pyriproxyfen 97% CAS 95737-68-1View Details
95737-68-1 -
 Pyriproxyfen CAS 95737-68-1View Details
Pyriproxyfen CAS 95737-68-1View Details
95737-68-1 -
 Pyriproxyfen CAS 95737-68-1View Details
Pyriproxyfen CAS 95737-68-1View Details
95737-68-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8