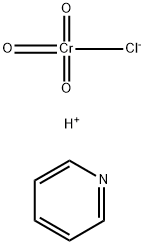
Pyridinium dichromate
Synonym(s):PDC
- CAS NO.:20039-37-6
- Empirical Formula: C5H7Cr2NO7
- Molecular Weight: 297.1
- MDL number: MFCD00013105
- EINECS: 243-478-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
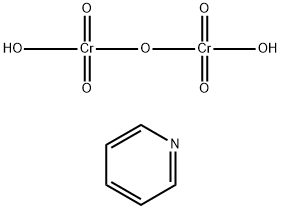
What is Pyridinium dichromate?
Description
Palladium on carbon (Pd/C) is a common catalyst for hydrogenation and/or hydrogenolysis of various functional groups. It is typically purchased as a black powder which is 5% or 10% palladium (by wt%) adsorbed on carbon. The reagent is normally purchased dry or as a solid which is 50% wet with water. The 50% wet with water reagent is the safer to handle option.
Description
Pyridinium dichromate (PDC) is a strong oxidizing agent. It is able to convert primary alcohols to aldehydes, and secondary alcohols to ketones. Because of the associated toxicity of the reagent it is currently rarely used.
Chemical properties
Orange solid
The Uses of Pyridinium dichromate
Pyridinium dichromate (PDC) is an orange colored solid used as an oxidizing agent.
Oxidizing agent for conversion of primary alcohols to aldehydes and ketones, acetals to esters, and didehydroketones to enones in the presence of tert-butyl hydroperoxide.
Pyridinium Dichromate may be used as an alternative to PCC in nucleoside and carbohydrate oxidation, particularly for fragile molecules. PDC can also be used in conjunction with tertbutylhydroperoxide for a variety of oxidative transformations.
The Uses of Pyridinium dichromate

A mixture of the SM (7.2 g, 26.2 mmol) and 10% Pd/C (2 g) in MeOH (100 mL) was stirred under an atmosphere of H2 (50 psi) at RT for 12 h. The mixture was filtered through celite and the filtrate was concentrated. The residue was purified by silica gel flash chromatography to provide the product. [5.8 g, 80%]
The Uses of Pyridinium dichromate
Pyridinium dichromate acts as a strong oxidizing agent used in the conversion of primary alcohols and secondary alcohols to aldehydes and ketones respectively. It plays an important role in the oxidation of unsaturated tertiary alcohols, silyl ethers, the carbon-boron bond, and oximes. Further, it is used in the conversion of acetals to esters and didehydroketones to enones in the presence of tert-butyl hydroperoxide.
Preparation
Pyridinium dichromate can be obtained by gradual addition of a solution of chromic anhydride (Cr2O3) in water to pyridine in ice cold conditions.
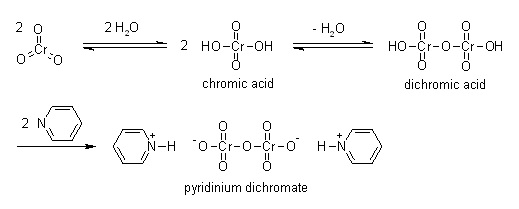
Definition
ChEBI: Pyridinium dichromate is a pyridinium salt that is the dipyridinium salt of dichromic acid. It is a strong oxidizing agent which can convert primary and secondary alcohols to aldehydes and ketones respectively. It has a role as an oxidising agent. It contains a dichromate(2-).
Flammability and Explosibility
Flammable
Purification Methods
Dissolve it in the minimum volume of H2O and add 5 volumes of cold Me2CO and cool to -20o. After 3hours the orange crystals are collected, washed with a little cold Me2CO and dried in a vacuum. It is soluble in dimethylformamide (0.9g/mL at 25o), and in H2O, and has a characteristic IR with max at 930, 875, 765, 730 and 730 cm-1. [Corey & Schmidt Tetrahedron Lett 399 1979, Coats & Corrigan Chem Ind (London) 1594 1969.] (Possible CARCINOGEN.) § Available commercially on a polymer support.
Properties of Pyridinium dichromate
| Melting point: | 152-153 °C(lit.) |
| Density | 1.71[at 20℃] |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble in DMSO |
| form | Crystalline Powder |
| appearance | Red/orange solid |
| appearance | Black powder |
| color | Orange to brown |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Exposure limits | OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| CAS DataBase Reference | 20039-37-6(CAS DataBase Reference) |
| EPA Substance Registry System | Chromic acid (H2Cr2O7), compd. with pyridine (1:2) (20039-37-6) |
Safety information for Pyridinium dichromate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H272:Oxidising liquids;Oxidising solids H314:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyridinium dichromate
| InChIKey | BNSOYWDFFBDEFB-UHFFFAOYSA-L |
Pyridinium dichromate manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 20039-37-6 98%View Details
20039-37-6 98%View Details
20039-37-6 -
 Pyridinium dichromate CAS 20039-37-6View Details
Pyridinium dichromate CAS 20039-37-6View Details
20039-37-6 -
 Pyridinium dichromate CAS 20039-37-6View Details
Pyridinium dichromate CAS 20039-37-6View Details
20039-37-6 -
 Pyridinium Dichromate CAS 20039-37-6View Details
Pyridinium Dichromate CAS 20039-37-6View Details
20039-37-6 -
 Pyridinium dichromate 98% CAS 20039-37-6View Details
Pyridinium dichromate 98% CAS 20039-37-6View Details
20039-37-6 -
 Pyridinium dichromate CAS 20039-37-6View Details
Pyridinium dichromate CAS 20039-37-6View Details
20039-37-6 -
 Pyridinium DichromateView Details
Pyridinium DichromateView Details
20039-37-6 -
 Powder White Crystal Pyridinium Dichromate CAS: 20039-37-6View Details
Powder White Crystal Pyridinium Dichromate CAS: 20039-37-6View Details
20039-37-6
