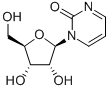
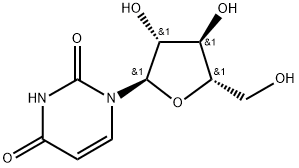
Pseudouridine
- CAS NO.:1445-07-4
- Empirical Formula: C9H12N2O6
- Molecular Weight: 244.2
- MDL number: MFCD00038458
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
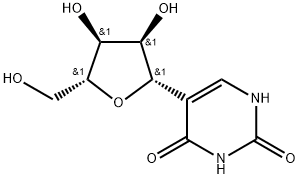
What is Pseudouridine?
Description
β-Pseudouridine is the C-5 glycoside isomer of the nucleoside uridine. It is formed when uridine in RNA undergoes site-specific isomerization by a pseudouridine synthase enzyme. Pseudouridine is found in tRNAs from bacteria, archaea, and eukaryotes. In vitro, it reduces the number of X-ray-induced chromosomal aberrations in human lymphocytes isolated from whole blood in a dose-dependent manner.
The Uses of Pseudouridine
An isomer of the nucleoside uridine found in all species and in many classes of RNA except mRNA. It is formed by enzymes called Ψ synthases, which post-transcriptionally isomerize specific uridine residues in RNA in a process termed pseudouridylation. Studies suggest that β-Pseudouridine reduces radiation-induced chromosome aberrations in human lymphocytes.
What are the applications of Application
β-Pseudouridine is a compound also known as 5-β-D-Ribofuranosylpyrimidine-2,4(1H,3H)-dione
Definition
ChEBI: Pseudouridine is a C-glycosyl pyrimidine that consists of uracil having a beta-D-ribofuranosyl residue attached at position 5. The C-glycosyl isomer of the nucleoside uridine. It has a role as a fundamental metabolite.
Origin
Pseudouridine (Ψ) was the first modified ribonucleotide discovered 7 decades ago, and it has been found in tRNA, rRNA, snRNA, mRNA, and other types of RNA[2].
Biochem/physiol Actions
Pseudouridine is essential for rRNA folding and for regulating translational accuracy and it is required for stabilizing the tRNA structure. Pseudouridine in rRNA and tRNA has been shown to fine-tune and stabilize the regional structure and help maintain their functions in mRNA decoding, ribosome assembly, processing and translation. Pseudouridine in snRNA has been shown to enhance spliceosomal RNA-pre-mRNA interaction to facilitate splicing regulation[1].
References
[1] Mingjia Chen, Claus-Peter Witte. “A Kinase and a Glycosylase Catabolize Pseudouridine in the Peroxisome to Prevent Toxic Pseudouridine Monophosphate Accumulation.” Plant Cell 32 3 (2020): 722–739.
[2] Pedro Morais, Yi-Tao Yu, Hironori Adachi. “The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines.” Frontiers in Cell and Developmental Biology (2021): 789427.
Properties of Pseudouridine
| Melting point: | 222 °C |
| Density | 1.641±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Methanol (Very Slightly, Heated), Water (Slightly, Sonicated, Heated) |
| form | Solid |
| pka | 8.52±0.10(Predicted) |
| color | White to Off-White |
| λmax | 264nm(MeOH)(lit.) |
| InChI | InChI=1/C9H12N2O6/c12-2-4-5(13)6(14)7(17-4)3-1-10-9(16)11-8(3)15/h1,4-7,12-14H,2H2,(H2,10,11,15,16)/t4-,5-,6-,7+/s3 |
Safety information for Pseudouridine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pseudouridine
| InChIKey | PTJWIQPHWPFNBW-IZFRNLNUNA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](CO)O[C@H]1C1=CNC(=O)NC1=O)O |&1:1,2,3,7,r| |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2,4(1H,3H)-Pyrimidinedione, 5-[5-O-[(1,1-dimethylethyl)diphenylsilyl]-β-D-ribofuranosyl]-](https://img.chemicalbook.in/)
![6-[(3-chloropropyl)amino]-1,3-dimethyluracil](https://img.chemicalbook.in/CAS/GIF/34654-81-4.gif)

You may like
-
 Pseudouridine (Synthetic) CAS 1445-07-4View Details
Pseudouridine (Synthetic) CAS 1445-07-4View Details
1445-07-4 -
 Pseudouridine CAS 1445-07-4View Details
Pseudouridine CAS 1445-07-4View Details
1445-07-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1