N-PROPYLBENZENE
Synonym(s):1-Phenylpropane;Isocumene
- CAS NO.:103-65-1
- Empirical Formula: C9H12
- Molecular Weight: 120.19
- MDL number: MFCD00009377
- EINECS: 203-132-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
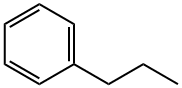
What is N-PROPYLBENZENE?
Chemical properties
colourless or light yellow liquid
Physical properties
Clear, colorless, mobile liquid with an odor similar to ethylbenzene or toluene. An odor threshold concentration of 3.8 ppbv was determined by a triangular odor bag method (Nagata and Takeuchi, 1990). Cometto-Mu?iz and Cain (1994) reported an average nasal pungency threshold concentration of 1,487 ppmv.
The Uses of N-PROPYLBENZENE
n-Propylbenzene is used to prepare benzoic acid. It is employed as a solvent for gas chromatography. It is also used as a solvent and an intermediate in organic synthesis. Further, it is used in fuels and fuel additives.
The Uses of N-PROPYLBENZENE
In textile dyeing and printing; as solvent for cellulose acetate.
Definition
ChEBI: An alkylbenzene that is benzene having one of its aromatic hydrogens substituted by a propyl group.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 3323, 1953 DOI: 10.1021/ja01110a009
The Journal of Organic Chemistry, 60, p. 2430, 1995 DOI: 10.1021/jo00113a024
Tetrahedron Letters, 28, p. 3817, 1987 DOI: 10.1016/S0040-4039(00)96393-7
General Description
A clear colorless liquid. Insoluble in water and less dense than water. Flash point 86°F. Mildly toxic by ingestion and inhalation. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as PROPYL BENZENE, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
Hazard
Flammable, moderate fire risk.
Health Hazard
May be harmful by inhalation, ingestion, or skin absorption. May cause eye and skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Vapor may travel considerable distance to a source of ignition and flashback.
Source
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average propylbenzene concentrations reported in water-soluble fractions of unleaded
gasoline, kerosene, and diesel fuel were 246, 82, and 23 μg/L, respectively. When the authors
analyzed the aqueous-phase via U.S. EPA approved test method 610, average propylbenzene
concentrations in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were
generally lower, i.e., 210, 57, and 25 μg/L, respectively.
Schauer et al. (1999) reported propylbenzene in a diesel-powered medium-duty truck exhaust at
an emission rate of 100 μg/km.
California Phase II reformulated gasoline contained propylbenzene at a concentration of 3.5
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 0.83 and 97.9 mg/km, respectively (Schauer et al., 2002).
Identified as one of 140 volatile constituents in used soybean oils collected from a processing
plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
Drinking water standard: No MCLGs or MCLs have been proposed (U.S. EPA, 1996).
Environmental Fate
Biological. A Nocardia sp., growing on n-octadecane, biodegraded propylbenzene to
phenylacetic acid (Davis and Raymond, 1981). Propylbenzene was cometabolized by a strain of
Micrococcus cerificans to cinnamic acid (Pitter and Chudoba, 1990).
Estimated half-lives of propylbenzene (0.8 μg/L) from an experimental marine mesocosm
during the spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 19, 1.3, and 11 d,
respectively (Wakeham et al., 1983).
Photolytic. A rate constant of 3.7 x 10-9 L/molecule?sec was reported for the reaction of
propylbenzene with OH radicals in the gas phase (Darnall et al., 1976). Similarly, a room
temperature rate constant of 5.7 x 10-12 cm3/molecule?sec was reported for the vapor-phase
reaction of propylbenzene with OH radicals (Atkinson, 1985). At 25 °C, a rate constant of 6.58 x
10-12 cm3/molecule?sec was reported for the same reaction (Ohta and Ohyama, 1985).
Chemical/Physical. Propylbenzene will not hydrolyze because it does not contain a
hydrolyzable functional group (Kollig, 1993).
Properties of N-PROPYLBENZENE
| Melting point: | -99 °C (lit.) |
| Boiling point: | 159 °C (lit.) |
| Density | 0.862 g/mL at 25 °C (lit.) |
| vapor density | 4.14 (vs air) |
| vapor pressure | 2 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 118 °F |
| storage temp. | 2-8°C |
| solubility | 0.06g/l |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Clear slightly yellow |
| Odor Threshold | 0.0038ppm |
| explosive limit | 0.8-6%(V) |
| Water Solubility | Miscible with alcohol, ether, acetone, benzene, and petroleum ether. Slightly miscible with water. |
| Merck | 14,7844 |
| BRN | 1903006 |
| Henry's Law Constant | 4.35, 6.21, 8.37, 11.6, and 15.3 at 10, 15, 20, 25, and 30 °C, respectively (headspace-GC, Perlinger
et al., 1993) |
| Dielectric constant | 2.4(20℃) |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 103-65-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, propyl-(103-65-1) |
| EPA Substance Registry System | n-Propylbenzene (103-65-1) |
Safety information for N-PROPYLBENZENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. |
Computed Descriptors for N-PROPYLBENZENE
| InChIKey | ODLMAHJVESYWTB-UHFFFAOYSA-N |
N-PROPYLBENZENE manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Propylbenzene CAS 103-65-1View Details
Propylbenzene CAS 103-65-1View Details
103-65-1 -
 Propylbenzene, 98% CAS 103-65-1View Details
Propylbenzene, 98% CAS 103-65-1View Details
103-65-1 -
 Propylbenzene 95% CAS 103-65-1View Details
Propylbenzene 95% CAS 103-65-1View Details
103-65-1 -
 Propylbenzene CAS 103-65-1View Details
Propylbenzene CAS 103-65-1View Details
103-65-1 -
 Propylbenzene CAS 103-65-1View Details
Propylbenzene CAS 103-65-1View Details
103-65-1 -
 Propylbenzene CAS 103-65-1View Details
Propylbenzene CAS 103-65-1View Details
103-65-1 -
 103-65-1 Propylbenzene 98%View Details
103-65-1 Propylbenzene 98%View Details
103-65-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
