Prednisolone-21-acetate
Synonym(s):1,4-Pregnadiene-11β,17α,21-triol-3,20-dione 21-acetate;11β,17α,21-Trihydroxy-1,4-pregnadiene-3,20-dione 21-acetate;21-Acetoxy-1,4-pregnadiene-11β,17α-diol-3,20-dione;Prednisolone 21-acetate
- CAS NO.:52-21-1
- Empirical Formula: C23H30O6
- Molecular Weight: 402.49
- MDL number: MFCD00037710
- EINECS: 200-134-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
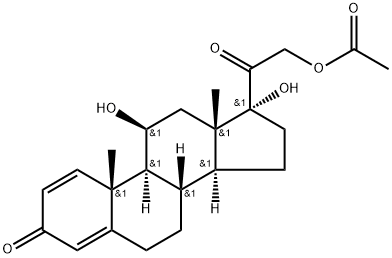
What is Prednisolone-21-acetate ?
Absorption
Prednisolone acetate oral suspension given at a dose equivalent to 15mg prednisolone has a Cmax of 321.1ng/hr, a Tmaxof 1-2 hours, and an AUC of 1999.4ng*hr/mL. The absorption pharmacokinetics of prednisolone acetate are not significantly different from a comparable dose of prednisolone.
Toxicity
The oral LD50 of prednisolone acetate in mice is 1680 mg/kg. Patients experiencing an overdose of oral prednisolone acetate may experience an increased severity in the adverse effects of corticosteroids. Overdose of oral prednisolone acetate may be treated by gastric lavage or inducing vomiting if the overdose was recent, as well as supportive and symptomatic therapy. Chronic overdosage may be treated by dose reduction or treating patients on alternate days. An overdose by the ophthalmic route is not expected to cause problems.
Chemical properties
White or almost white, crystalline powder.
Originator
Sterane, Phipharmex ,US ,1955
The Uses of Prednisolone-21-acetate
Synthetic corticosteroid; metabolically interconvertible with prednisone.
The Uses of Prednisolone-21-acetate
glucocorticoid
Background
Prednisolone acetate is a prednisolone molecule bound to an acetate functional group by an ester bond.
Prednisolone acetate was granted FDA approval in 1955.
Indications
Prednisolone acetate is indicated as an anti-inflammatory or immunosuppressive agent for allergic, dermatologic, gastrointestinal, hematologic, ophthalmologic, nervous system, renal, respiratory, rheumatologic, or infectious conditions. Prednisolone acetate is also indicated in organ transplant patients, as well as endocrine or neoplastic conditions.
What are the applications of Application
Prednisolone 21-acetate is corticosteroid that is metabolically interconvertible with prednisone
Definition
ChEBI: Prednisolone acetate is a corticosteroid hormone.
Manufacturing Process
To a solution of 0.85 gram of 1,4-pregnadiene-11β,17α,21-triol-3,20-dione (prednisolone) in 5 ml of pyridine are added 3 ml of acetic anhydride. The reaction mixture is allowed to stand at room temperature overnight and is then diluted with ice water. The resulting precipitate is filtered from the mixture and recrystallized from acetone-hexane. There is recovered 0.45 gram of 1,4-pregnadiene-11β,17α,21-triol-3,20-dione 21-acetate, MP 235°239°C. On recrystallization, the MP rose to 237°-239°C.
brand name
Econopred (Alcon); Meticortelone (Schering); Omnipred (Alcon); Sterane (Pfizer).
Therapeutic Function
Glucocorticoid
Biological Activity
Prednisolone Acetate (Omnipred) is a synthetic corticosteroid that is a particularly potent immunosuppressant.
Pharmacokinetics
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Prednisolone acetate has a short duration of action as the half life is 2-3 hours. Corticosteroids have a wide therapeutic window as patients make require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Clinical Use
For rheumatoid arthritis, rheumatic fever, lupus erythematosus, scleroderma, dermatomyositis, acute lymphoblastic leukemia, etc.
Metabolism
Prednisolone acetate undergoes ester hydrolysis to prednisolone. After this step, the drug undergoes the normal metabolism of prednisolone.
Prednisolone can be reversibly metabolized to prednisone which is then metabolized to 17α,21-dihydroxy-pregnan-1,4,6-trien-3,11,30-trione (M-XVII), 20α-dihydro-prednisone (M-V), 6βhydroxy-prednisone (M-XII), 6α-hydroxy-prednisone (M-XIII), or 20β-dihydro-prednisone (M-IV). 20β-dihydro-prednisone is metabolized to 17α,20ξ,21-trihydroxy-5ξ-pregn-1-en-3,11-dione(M-XVIII). Prednisolone is metabolized to Δ6-prednisolone (M-XI), 20α-dihydro-prednisolone (M-III), 20β-dihydro-prednisolone (M-II), 6αhydroxy-prednisolone (M-VII), or 6βhydroxy-prednisolone(M-VI). 6αhydroxy-prednisolone is metabolized to 6α,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-X). 6βhydroxy-prednisolone is metabolized to 6β,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-VIII), 6β,11β,17α,20α,21-pentahydroxypregnan-1,4-diene-3-one (M-IX), and 6β,11β,17α,21-tetrahydroxy-5ξ-pregn-1-en-3,20-dione (M-XIV). MVIII is metabolized to 6β,11β,17α,20β,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XV) and then to MXIV, while MIX is metabolized to 6β,11β,17α,20α,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XVI) and then to MXIV. These metabolites and their glucuronide conjugates are excreted predominantly in the urine.
Purification Methods
Recrystallise prednisolone acetate from EtOH, Me2CO, Me2CO/hexane, and it has UV with max at 243nm in EtOH. [Joly et al. Bull Soc Chim Fr 366 1958; Herzog et al. J Am Chem Soc 77 4781 1955, Beilstein 8 IV 3468.]
Properties of Prednisolone-21-acetate
| Melting point: | 240-244 °C |
| Boiling point: | 579.8±50.0 °C(Predicted) |
| alpha | D25 +116° (dioxane) |
| Density | 1.28±0.1 g/cm3(Predicted) |
| refractive index | 112 ° (C=1, Dioxane) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, slightly soluble in ethanol (96 per cent) and in methylene chloride. |
| form | neat |
| pka | 12.41±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 11.6mg/L(25 ºC) |
| Merck | 7721 |
| BRN | 3111798 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 52-21-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Pregnadiene-3,20-dione, 11beta,17alpha,21-trihydroxy-, acetate(52-21-1) |
Safety information for Prednisolone-21-acetate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Prednisolone-21-acetate
Prednisolone-21-acetate manufacturer
Omicron Pharmatech Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
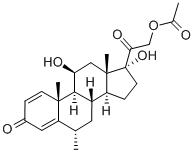




You may like
-
 Prednisolone Acetate 99%View Details
Prednisolone Acetate 99%View Details -
 Prednisolone 21-acetate 99%View Details
Prednisolone 21-acetate 99%View Details -
 52-21-1 99%View Details
52-21-1 99%View Details
52-21-1 -
 Prednisolone acetate Sterile 98%View Details
Prednisolone acetate Sterile 98%View Details -
 Prednisolone acetate 95.00% CAS 52-21-1View Details
Prednisolone acetate 95.00% CAS 52-21-1View Details
52-21-1 -
 Prednisolone 21-acetate CAS 52-21-1View Details
Prednisolone 21-acetate CAS 52-21-1View Details
52-21-1 -
 Prednisolone Acetate CAS 52-21-1View Details
Prednisolone Acetate CAS 52-21-1View Details
52-21-1 -
 Prednisolone acetate CAS 52-21-1View Details
Prednisolone acetate CAS 52-21-1View Details
52-21-1
