2-Benzothiazolamine
Synonym(s):2-Aminobenzothiazole;2-Benzothiazolamine;2-Benzothiazolylamine
- CAS NO.:136-95-8
- Empirical Formula: C7H6N2S
- Molecular Weight: 150.2
- MDL number: MFCD00005785
- EINECS: 205-268-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-07 19:09:50
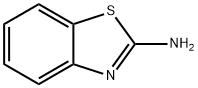
What is 2-Benzothiazolamine?
Chemical properties
BEIGE TO GREYISH POWDER OR FLAKES
The Uses of 2-Benzothiazolamine
An impurity of pramipexole production
The Uses of 2-Benzothiazolamine
2-Aminobenzothiazole was used in the synthesis of cobalt(II) picrate mixed-ligand complexes. It was used to study adsorption of biologically significant 2-aminobenzothiazole molecules on colloidal silver particles using surface-enhanced raman scattering spectroscopy.
Definition
ChEBI: 2-aminobenzothiazole is a member of benzothiazoles.
General Description
Odorless gray to white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine, organosulfide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for 2-Benzothiazolamine are not available; however, 2-Benzothiazolamine is probably combustible.
Biochem/physiol Actions
2-Aminobenzothiazole has local anaesthetic action and has numerous applications in human and veterinary medicine.
Purification Methods
The thiazole cystallises from a H2O, aqueous EtOH, *C6H6 or pet ether. The hydrochloride crystallises from dilute HCl and has m 240.5o. [Beilstein 27 H 182, 27 III/IV 4824.]
Properties of 2-Benzothiazolamine
| Melting point: | 126-129 °C (lit.) |
| Boiling point: | 190-195 °C(Press: 0.05 Torr) |
| Density | 1.2162 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | Soluble in alcohol, chloroform, diethyl ether. |
| pka | pK1: 4.48(+1) (20°C) |
| form | Powder or Flakes |
| color | Beige to grayish |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Merck | 14,424 |
| BRN | 116315 |
| CAS DataBase Reference | 136-95-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzothiazole, 2-amino-(136-95-8) |
| EPA Substance Registry System | 2-Aminobenzothiazole (136-95-8) |
Safety information for 2-Benzothiazolamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2-Benzothiazolamine
2-Benzothiazolamine manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
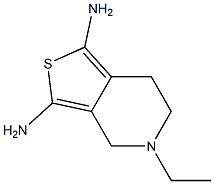
![2,6-diamino-4,5,6,7-tetrahydrobenzo[d]thiazol-7-ol](https://img.chemicalbook.in/CAS/20180527/GIF/1001648-75-4.gif)
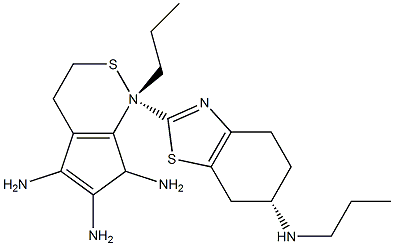
![N6-propyl-3a,4,5,6,7,7a-hexahydrobenzo[d]thiazole-2,6-diamine](https://img.chemicalbook.in/CAS/20180629/GIF/1052691-22-1.gif)

You may like
-
 102337-98-4 136-95-8 94787-08-3 1,3-benzothiazol-2-ylamine 98%View Details
102337-98-4 136-95-8 94787-08-3 1,3-benzothiazol-2-ylamine 98%View Details
102337-98-4 136-95-8 94787-08-3 -
 2-Aminobenzothiazole 98%View Details
2-Aminobenzothiazole 98%View Details
136-95-8 -
 2-Aminobenzothiazole 136-95-8 98%View Details
2-Aminobenzothiazole 136-95-8 98%View Details
136-95-8 -
 136-95-8 2-Aminobenzothiazole, 97% 99%View Details
136-95-8 2-Aminobenzothiazole, 97% 99%View Details
136-95-8 -
 2-Aminobenzothiazole CAS 136-95-8View Details
2-Aminobenzothiazole CAS 136-95-8View Details
136-95-8 -
 2-Aminobenzothiazole CAS 136-95-8View Details
2-Aminobenzothiazole CAS 136-95-8View Details
136-95-8 -
 2-Aminobenzothiazole CAS 136-95-8View Details
2-Aminobenzothiazole CAS 136-95-8View Details
136-95-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
