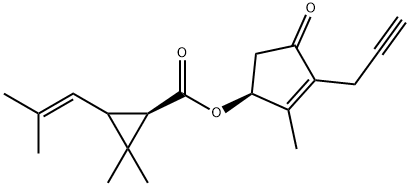
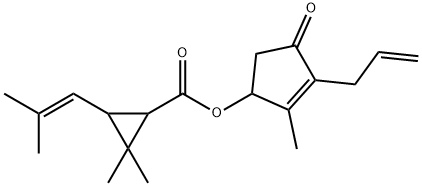
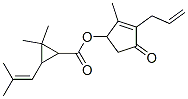
Prallethrin
Synonym(s):(RS)-2-Methyl-4-oxo-3-prop-2-ynylcyclopent-2-enyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
- CAS NO.:23031-36-9
- Empirical Formula: C19H24O3
- Molecular Weight: 300.39
- MDL number: MFCD01632766
- EINECS: 245-387-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
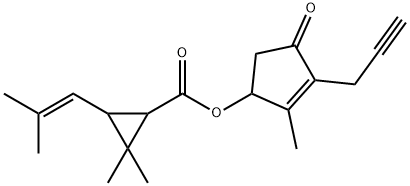
What is Prallethrin?
Chemical properties
solid
The Uses of Prallethrin
Prallethrin ia a synthetic pyrethroid; propynyl analog of allethrin. Prallethrin is used as an insecticide.
The Uses of Prallethrin
Prallethrin is used for the control of insects in domestic and public health situations.
Definition
ChEBI: Prallethrin is a member of cyclopropanes and a terminal acetylenic compound. It has a role as a pyrethroid ester insecticide and an agrochemical. It is functionally related to a chrysanthemic acid.
Hazard
A poison by ingestion and inhalation. Low toxicity by skin contact.
Pharmacology
Prallethrin is a propynyl analog of allethrin, with 2-methyl-4-oxo-3-(prop-2-ynyl)-cyclopent-2-enyl as the secondary alcohol moiety that has been introduced against public health pests (113–115). The ester of the (S)-alcohol with (1R)-trans,cis-chrysanthemic acid resolved partially is commercialized on the strength of the assessment of insecticidal action of individual stereoisomers (116). Compared with d-allethrin, it is approximately four, six, and five times more effective on Musca domestica (for killing), Blattella germanica (for killing), and Culex pipiens pallens (for knockdown), respectively (43). A lower level of resistance to prallethrin than to phenoxybenzyl pyrethroids was demonstrated in a kdr-resistant housefly strain (116).
Safety Profile
A poison by ingestion and inhalation. Low toxicity by skin contact. When heated to decomposition it emits acrid smoke and irritating vapors.
Metabolic pathway
Prallethrin is stable under normal room temperature storage conditions for up to 36 months. It is labile to alkali at the ester linkage, forming 2 and 3 (Scheme 1). Analogy with the allethrins (from which it differs only in the degree of unsaturation in the side chain - propyne rather than propene) would suggest that the compound is very photolabile.
Degradation
The metabolism of prallethrin was studied following single oral administration
and subcutaneous injection of the cis- and trans-isomers to rats at
two dose levels and after repeated dosing. The compound was labelled in
the alcohol moiety (inferred from the results) (PSD, 1995).
Absorption was rapid with maximum tissue residues being attained
3 hours after the dosing of each isomer. Biphasic clearance of radioactivity
then occurred with half-lives of 3 and 14-23 hours (cis-isomer) and 3-5
and 7-35 hours (trans-isomer). Urinary excretion accounted for 13-32%
(cis-isomer) and 45-62% (trans-isomer) of the dose with most of the balance in the faeces. Less than 0.1% was exhaled as 14CO2 . Residues
in tissues were very low (0.3% of the dose at 7 days). Metabolism and
disposition were independent of dose and dose route but residues in
tissues were somewhat higher for the cis-isomer.
Twenty-one metabolites were identified (taking account of stereochemistry
and conjugates). The major pathways of metabolism involved
oxidation at a methyl group of the isobutenyl group in the acid moiety
(giving 4 and 5), at the C1 and C2 positions of the propynyl group in the
alcohol moiety (giving 6 and 9) and dihydroxylation (to 7 and 8). The
resulting hydroxy derivatives were conjugated with glucuronic acid and
sulfate.
Hydrolysis of prallethrin also occurred to afford the acid 2 (presumed)
and the alcohol 3. Hydrolysis of the hydroxylated prallethrins and further
oxidation of 3 afforded 10 and 11. Further oxidation of 10 to the cyclic
tertiary alcohol (12) was observed.
When bluegill sunfish were exposed to a l4C-labelled (acid and alcohol
groups) prallethrin isomer (1Rtrans), more than 50% of the biliary
radioactivity recovered from the gall bladder was observed as one ester metabolite. This was identified as the taurine conjugate of carboxylic acid
metabolite 5 (Oshima et al., 1992).
Properties of Prallethrin
| Melting point: | 25°C |
| Boiling point: | 381.62°C (rough estimate) |
| Density | d420 1.03 |
| vapor pressure | ﹤4.3×10-5 Pa (23.1 °C) |
| refractive index | 1.4200 (estimate) |
| Flash point: | 139 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Sparingly), Ethyl Acetate (Slightly), Methanol (Sparingly) |
| form | neat |
| Water Solubility | 8 mg l-1 (25 °C) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 23031-36-9(CAS DataBase Reference) |
| EPA Substance Registry System | Prallethrin (23031-36-9) |
Safety information for Prallethrin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H331:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Prallethrin
Prallethrin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 23031-36-9 Prallethrin 98%View Details
23031-36-9 Prallethrin 98%View Details
23031-36-9 -
 23031-36-9 98%View Details
23031-36-9 98%View Details
23031-36-9 -
 Prallethrin 98%View Details
Prallethrin 98%View Details
23031-36-9 -
 Prallethrin 95.00% CAS 23031-36-9View Details
Prallethrin 95.00% CAS 23031-36-9View Details
23031-36-9 -
 Prallethrin CAS 23031-36-9View Details
Prallethrin CAS 23031-36-9View Details
23031-36-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1