Potassium thioacetate
Synonym(s):Ethanethioic acid potassium salt;Potassium thioacetate;Thioacetic acid potassium salt;Thiolacetic acid potassium salt
- CAS NO.:10387-40-3
- Empirical Formula: C2H4OS.K
- Molecular Weight: 115.22
- MDL number: MFCD00137704
- EINECS: 233-848-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 16:23:17
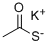
What is Potassium thioacetate?
Chemical properties
white to light brown crystalline powder, crystals
The Uses of Potassium thioacetate
Potassium Thioacetate is the potassium salt of Thioacetic Acid, a commonly used reagent in organic synthesis for the introduction of thiol groups in molecules.
The Uses of Potassium thioacetate
Potassium thioacetate is used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives. It is also used as a reagent in the conversion of halides to thiols.
What are the applications of Application
Potassium thioacetate is a thiol releasing reagent in halide preparation
What are the applications of Application
Potassium thioacetate is an organosulfur compound and a salt with the formula CH3COS−K+. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives . It acts as a sulfur source in the synthesis of sulfur-containing organic compounds for the synthesis of heterocycles, polymers, transition-metal ligands, nanoparticles, bioactive compounds and macromolecular inclusion complexes. It is also used for palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives and it is also used as a reagent in the conversion of halides to thiols.
Reactions
Thioacetate is also a class of sulfur-containing nucleophiles, of which potassium thioacetate is the most widely used reagent. Potassium thioacetate reacts with organic halides to form thioesters, which are often used as thiol-protecting groups.In the classical reaction, potassium thioacetate replaces the bromine atom to form a thiol in which the thioester formed in the first step is subjected to a nucleophilic addition elimination reaction to obtain a thiol via hydrolysis, alcoholysis or ammonolysis. Unlike the thiol formation from bromine and thiourea, this method is not strictly limited to polystyrenes. It can also be extended to poly(meth)acrylate systems. It is important that there is no observation of ester hydrolysis during the process.
Properties of Potassium thioacetate
| Melting point: | 173-176 °C (lit.) |
| Density | 1.58 g/cm3 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble |
| form | Crystalline Powder, Crystals or Chunks |
| color | White to light brown |
| Water Solubility | soluble |
| Sensitive | Air Sensitive & Hygroscopic |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| BRN | 3595448 |
| CAS DataBase Reference | 10387-40-3(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanethioic acid, potassium salt (10387-40-3) |
Safety information for Potassium thioacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Potassium thioacetate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 10387-40-3 98%View Details
10387-40-3 98%View Details
10387-40-3 -
 Potassium Thio Acetate 98%View Details
Potassium Thio Acetate 98%View Details
10387-40-3 -
 S-Potassium Thioacetate CAS 10387-40-3View Details
S-Potassium Thioacetate CAS 10387-40-3View Details
10387-40-3 -
 Potassium thioacetate, 98% CAS 10387-40-3View Details
Potassium thioacetate, 98% CAS 10387-40-3View Details
10387-40-3 -
 Potassium thioacetate CAS 10387-40-3View Details
Potassium thioacetate CAS 10387-40-3View Details
10387-40-3 -
 Potassium thioacetate 98% CAS 10387-40-3View Details
Potassium thioacetate 98% CAS 10387-40-3View Details
10387-40-3 -
 Potassium thioacetate 95% CAS 10387-40-3View Details
Potassium thioacetate 95% CAS 10387-40-3View Details
10387-40-3 -
 Potassium thioacetate CAS 10387-40-3View Details
Potassium thioacetate CAS 10387-40-3View Details
10387-40-3
