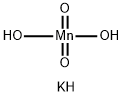
POTASSIUM MANGANATE
- CAS NO.:10294-64-1
- Empirical Formula: H3KMnO4
- Molecular Weight: 161.06
- MDL number: MFCD00043089
- EINECS: 233-665-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is POTASSIUM MANGANATE?
Chemical properties
Potassium manganate, K2MnO4, green solid, soluble, permanent in alkali, formed by heating to high temperature manganese dioxide and potassium carbonate, and then extracting with water, and evaporating the solution. The first step in the preparation of potassium manganate and permanganate from pyrolusite.
The Uses of POTASSIUM MANGANATE
Bleaching skins, fibers, oils; disinfectants; mordant (wool); batteries; photography; printing; source of oxygen (dyeing); water purification; oxi- dizing agent.
The Uses of POTASSIUM MANGANATE
It serves as an oxidizing agent.
The Uses of POTASSIUM MANGANATE
A selective oxidizing agent.
Hazard
Dangerous fire risk in contact with organic materials, strong oxidizing agent.
Properties of POTASSIUM MANGANATE
| Melting point: | 190 °C(lit.) |
| solubility | soluble in H2O; reacts with HCl |
| form | Powder |
| color | green crystals, crystalline |
| Water Solubility | Soluble in water |
| Merck | 14,7644 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| Stability: | Stable. Oxidizer - contact with combustible material may lead to fire. Protect from moisture. Incompatible with zinc, finely powdered metals, peroxides, strong reducing agents. |
| EPA Substance Registry System | Manganic acid (H2MnO4), dipotassium salt (10294-64-1) |
Safety information for POTASSIUM MANGANATE
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for POTASSIUM MANGANATE
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
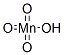

You may like
-
 Potassium manganate CAS 10294-64-1View Details
Potassium manganate CAS 10294-64-1View Details
10294-64-1 -
 Potassium manganate CAS 10294-64-1View Details
Potassium manganate CAS 10294-64-1View Details
10294-64-1 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
