Potassium hexafluorozirconate
Synonym(s):Potassium fluorozirconate;Potassium fluorozirconate (K2 ZrF6 );Potassium hexafluorozirconate (K2 ZrF6 );Potassium hexafluorozirconate(IV);Potassium zirconium fluoride (K2 ZrF6 )
- CAS NO.:16923-95-8
- Empirical Formula: F6K2Zr
- Molecular Weight: 283.41
- MDL number: MFCD00042547
- EINECS: 240-985-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
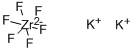
What is Potassium hexafluorozirconate?
Chemical properties
White crystals. Soluble in water (hot).
The Uses of Potassium hexafluorozirconate
Dipotassium hexafluorozirconate is used in the production metallic Zirconium.
The Uses of Potassium hexafluorozirconate
For manufacture of metallic zirconium.
General Description
A white crystalline solid. The primary hazard Potassium hexafluorozirconate the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Potassium hexafluorozirconate is slightly soluble in water. Potassium hexafluorozirconate is used in metal processing, as a catalyst in chemical manufacture, and for other uses.
Air & Water Reactions
Soluble in hot water [Hawley].
Reactivity Profile
Acidic inorganic salts, such as Potassium hexafluorozirconate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
INHALATION: Irritation, nose bleed possible. SKIN: May cause granulomatous reactions. INGESTION: Burning pain in the mouth and throat, vomiting, watery or bloody diarrhea, retching anuria, liver damage and collapse.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion. When heated to decomposition it emits toxic fumes of F and K2O. See also FLUORIDES and ZIRCONIUM COMPOUNDS.
Purification Methods
Recrystallise it from hot water (solubility is 0.78% at 2o and 25% at 100o).
Properties of Potassium hexafluorozirconate
| Melting point: | 840°C |
| Density | 3,5 g/cm3 |
| form | powder |
| color | colorless monoclinic crystals, crystalline |
| Water Solubility | Slightly soluble in cold water, soluble in hot water |
| Merck | 14,7639 |
| Solubility Product Constant (Ksp) | pKsp: 3.3 |
| Exposure limits | ACGIH: TWA 5 mg/m3; TWA 2.5 mg/m3; STEL 10 mg/m3 NIOSH: IDLH 25 mg/m3; IDLH 250 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 |
| CAS DataBase Reference | 16923-95-8(CAS DataBase Reference) |
| EPA Substance Registry System | Zirconium potassium fluoride (16923-95-8) |
Safety information for Potassium hexafluorozirconate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium hexafluorozirconate
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

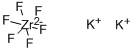
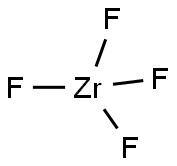

You may like
-
 Potassium hexafluorozirconate 98%View Details
Potassium hexafluorozirconate 98%View Details -
 16923-95-8 98%View Details
16923-95-8 98%View Details
16923-95-8 -
 16923-95-8 98%View Details
16923-95-8 98%View Details
16923-95-8 -
 Potassium Hexafluorozirconate pure CAS 16923-95-8View Details
Potassium Hexafluorozirconate pure CAS 16923-95-8View Details
16923-95-8 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
