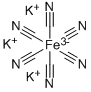
Potassium ferrocyanide trihyrate
- CAS NO.:14459-95-1
- Empirical Formula: C6H2FeKN6O-3
- Molecular Weight: 269.07
- MDL number: MFCD00167023
- EINECS: 680-418-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 18:06:30

What is Potassium ferrocyanide trihyrate?
Chemical properties
Lemon yellow monoclinic columnar crystal or powder, sometimes with cubic metamorphosis. Soluble in water, insoluble in ethanol, ether, methyl acetate and liquid ammonia.
The Uses of Potassium ferrocyanide trihyrate
Applied in formation of a xerogel via a cyanogel the gel has potential use in solid-state gas sensors.1
The Uses of Potassium ferrocyanide trihyrate
Moderately strong oxidizer when coupled with ferricyanide. Applied in formation of a xerogel via a cyanogel the gel has potential use in solid-state gas sensors.
The Uses of Potassium ferrocyanide trihyrate
Potassium ferrocyanide is a yellow crystal also known as yellow prussiate of potash. It was made by stirring hot potassium carbonate with wool or horn clippings with an iron rod. It is soluble in water 1:4 but not in alcohol. Potassium ferrocyanide was used as a developer for some iron processes and as an additive for alkaline pyro developers.
What are the applications of Application
Potassium hexacyanoferrate(II) trihydrate is a salt of the reducing ferrocyanide ion
Preparation
Potassium hexacyanoferrate(II), K4Fe(CN)6·3H20, is obtained commercially from gasworks spent oxide (which contains Prussian blue) by treatment with lime and subsequent treatment of the solution with a soluble potassium salt ; the less soluble potassium hexacyanoferrate(II) can be crystallized from this mixture. In the laboratory it is readily prepared by treating aqueous iron(II) sulphate with an excess of potassium cyanide ; the initially precipitated iron(II) cyanide dissolves upon boiling in the excess of potassium cyanide solution and the ferrocyanide crystallizes on cooling.
What are the applications of Application
Potassium hexacyanoferrate(II) trihydrate may be used in the following processes:
Preparation of Carrez I solution.
Milk sample preparation for gold nanoparticles (AuNPs) based colorimetric detection of melamine.
Preparation of ferrocyanide/ferricyanide reaction, a redox pair solution for electrochemical testing.
General Description
Potassium hexacyanoferrate(II) trihydrate, commonly known as potassium ferrocyanide, is a commonly used anticaking agent. Its cytotoxic and genotoxic effect has been evaluated. The growth of chemical garden in aqueous potassium hexacyanoferrate solution droplets on a superhydrophobic surface has been reported. The inhibition efficiency of potassium ferrocyanide in phosphoric acid solution against the corrosion of mild steel has been investigated.
Purification Methods
The anhydrous salt is prepared by drying at 110o over P2O5 in a vacuum desiccator. To obtain the trihydrate, it is necessary to equilibrate the salt in a desiccator over a saturated aqueous solution of sucrose and NaCl. It can also be precipitated from a saturated solution at 0o by adding an equal volume of cold 95% EtOH, setting aside for several hours, then centrifuge and wash with cold 95% EtOH. It is finally sucked air dry with water-pump vacuum. The anhydrous salt is obtained by drying the hydrate in a platinum boat at 90o in a slow stream of N2 [Loftfield & Swift J Am Chem Soc 60 3083 1938].
Structure and conformation
The trihydrate is diamagnetic and dimorphic existing in monoclinic (pseudotetragonal) and tetragonal forms. Its infrared spectrum in solution shows a single ν(C≡ N) at 2044 cm-1, but in the solid state a large number of bands are observed160; ν(Fe-C) occurs at 416cm-1. White, anhydrous K4Fe(CN)6 is obtained by dehydrating the trihydrate at 100°.
Properties of Potassium ferrocyanide trihyrate
| Melting point: | 70 °C(lit.) |
| Density | 1.85 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, yellow |
| form | fine crystals |
| appearance | Light yellow crystalline granules |
| color | Yellow |
| Specific Gravity | 1.85 |
| PH Range | 8 - 10 at 211 g/l at 25 °C |
| PH | 9.5 (100g/l, H2O, 20℃)(anhydrous substance) |
| Odor | Odorless |
| Water Solubility | 270 g/L (12 ºC) |
| Merck | 14,7631 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable. Incompatible with oxidizing agents, stong acids (may generate very toxic HCN). Not combustible. |
| CAS DataBase Reference | 14459-95-1(CAS DataBase Reference) |
Safety information for Potassium ferrocyanide trihyrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Potassium ferrocyanide trihyrate
Potassium ferrocyanide trihyrate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Potassium ferrocyanide 98%View Details
Potassium ferrocyanide 98%View Details -
 Potassium ferrocyanide trihydrate 98%View Details
Potassium ferrocyanide trihydrate 98%View Details -
 Potassium hexacyanoferrate(II) trihydrate, For ACS analysis; Begins to lose water at 60°, anhydrous at 100° CAS 14459-95-1View Details
Potassium hexacyanoferrate(II) trihydrate, For ACS analysis; Begins to lose water at 60°, anhydrous at 100° CAS 14459-95-1View Details
14459-95-1 -
 Potassium hexacyanoferrate(II) trihydrate CAS 14459-95-1View Details
Potassium hexacyanoferrate(II) trihydrate CAS 14459-95-1View Details
14459-95-1 -
 Potassium hexacyanoferrate(II) trihydrate, For ACS analysis; Begins to lose water at 60°, anhydrous at 100° CAS 14459-95-1View Details
Potassium hexacyanoferrate(II) trihydrate, For ACS analysis; Begins to lose water at 60°, anhydrous at 100° CAS 14459-95-1View Details
14459-95-1 -
 Potassium hexacyanoferrate(II) trihydrate 98.00% CAS 14459-95-1View Details
Potassium hexacyanoferrate(II) trihydrate 98.00% CAS 14459-95-1View Details
14459-95-1 -
 Potassium ferrocyanide, 99% CAS 14459-95-1View Details
Potassium ferrocyanide, 99% CAS 14459-95-1View Details
14459-95-1 -
 Potassium ferrocyanide, GR 99%+ CAS 14459-95-1View Details
Potassium ferrocyanide, GR 99%+ CAS 14459-95-1View Details
14459-95-1
