Polymethylhydrosiloxane
- CAS NO.:9004-73-3
- Empirical Formula: C5H11OSi*
- Molecular Weight: 115.22574
- MDL number: MFCD00084478
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 17:10:30
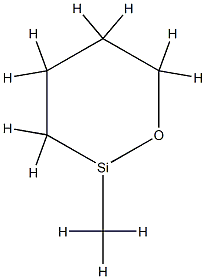
What is Polymethylhydrosiloxane?
Description
Methylpolysilicones (also called dimethylpolysiloxanes) are
high-molecular weight organic compounds of silicon which are
similar in chemical structure to inorganic silicates. They are members
of a large family in which silicon and oxygen atoms form a
siloxy skeleton to which various alkyl and aryl groups are attached
in regular, repetitive fashion. The methylpolysilicones may be
characterized by the following formula:
Si(CH3)3O[Si(CH3)2O] nSi(CH3)3
where "n" can be varied from 0 to 10,000 or higher.
The commercial methylpolysilicones are usually linear polymers,
although cyclic forms may also be utilized.
Methylpolysilicones can be prepared in viscosity grades ranging
from one to several hundred thousand centistokes (cs) depending
on the degree of polymerization. Those employed in food uses
generally have "n" values of 200-400, with molecular weights
usually between 14,000 and 21,000. In this molecular weight
range, methylpolysilicones are clear, colorless, viscous liquids,
which may contain up to 5% silicon dioxide. With higher degrees
of polymerization, resins and rubbers are produced. The methylpolysilicones
are insoluble in water and the lower alcohols, but
soluble in most aliphatic and aromatic hydrocarbon solvents. The
polymers are nonionic, inert compounds strongly resistant to
chemical and thermal attack. They are hydrophobic, greasy, and
markedly antiadhesive.
silica gel as well as emulsifying agents and preservatives. Foodgrade
methylpolysilicones must meet the following specifications:
Refractive index: 1.400 to 1.404.
Specific gravity: 0.964 to 0.973.
Viscosity: 300 to 600 cs.
Loss on heating: Not more than 18%.
Limit of impurities: Arsenic, not more than 3 parts per million
(ppm); heavy metals, not more than 10 ppm (expressed as lead).
The antifoaming and hydrophobic properties of dimethylpolysilicones
are also utilized medically in patients suffering from
excessive gas production or retention such as flatulence, gastric
bloating, or postoperative gaseous distention. They have also been
used to reduce gas shadows in radiography of the bowel and to
improve visualization in gastroscopy. The usual therapeutic adult
dose is 150-400 mg daily. Silicones are also employed to prevent
dermal irritation in bedridden patients. Medical grade preparations
(simethicone) must contain 93-99% dimethylpolysilicone, not
more than 4.5% silicon dioxide, and have a viscosity not less than
300 cs.
Because of their chemical and thermal resistance together with
their antiadhesive and hydrophobic properties, methylpolysilicones
have found various uses in the food industry. They are used
to coat containers and molds for bakery products, candies, and
confections; to impregnate packaging and wrapping paper which
contact foods; and to reduce foaming in various fermentation and
canning processes. Methylpolysilicones at low concentrations
(0.03-0.05 ppm) reduce thermal and oxidative deterioration of frying
fat by forming a protective film at the oil-air interface. At
higher levels (1-10 ppm), silicones added to shortening and other
frying fats may eliminate smoking difficulties by raising the smoke
point 20-30°F. Silicones have also been incorporated into cellulosic
sausage casings to facilitate the casing removal from the
enclosed meat mass.
Chemical properties
clear colorless viscous liquid
Physical properties
colorless free flowing liquid; average molecular weight 1500-2200 g mol?1 (supplier dependent); effective mass per hydride of 60 g mol?1; d = 1.006.
The Uses of Polymethylhydrosiloxane
Polymethylhydrosiloxane (PMHS) is an easily handled, inexpensive, non-toxic, and mild reducing agent. Although relatively inert towards organic functionality, PMHS can transfer its hydride to a variety of metal catalysts (including Sn, Ti, Zn, Cu, and Pd) which can then participate in a wide range of reductions. Alternatively, when made hypercoordinate by the action of fluoride or other nucleophiles, PMHS can act directly as a reducing agent. Polymethylhydrosiloxane is widely used as reducing agent often used in conjunction with metal catalysts or nucleophilic activators.
The Uses of Polymethylhydrosiloxane
methicone is a type of silicone used primarily in the formulation of free-flowing cosmetic powders. Methicone can also be found in cosmetic preparations as a skin surface sealant to reduce transepidermal water loss.
Preparation
hydrolysis of methyldichlorosilane followed by heating (60–150 °C) the resultant mixture of cyclic silanes in the presence of hexamethyldisiloxane generates the linear polysiloxane.
Properties of Polymethylhydrosiloxane
| Boiling point: | >177 °C(lit.) |
| Density | 1.006 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 250 °F |
| solubility | most ethereal, chlorinated, or hydrocarbon solvents as
well as EtOH, i-PrOH, warm DMF, and warm NMP; insoluble
in MeOH, DMSO, acetonitrile, and water. |
| form | Viscous Liquid |
| color | Clear colorless |
| Water Solubility | PRACTICALLY INSOLUBLE |
| Sensitive | Moisture Sensitive |
| CAS DataBase Reference | 9004-73-3(CAS DataBase Reference) |
| EPA Substance Registry System | Poly[oxy(methylsilylene)] (9004-73-3) |
Safety information for Polymethylhydrosiloxane
Computed Descriptors for Polymethylhydrosiloxane
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

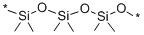

You may like
-
 Polymethylhydrosiloxane CAS 9004-73-3View Details
Polymethylhydrosiloxane CAS 9004-73-3View Details
9004-73-3 -
 Polymethylhydrosiloxane CAS 9004-73-3View Details
Polymethylhydrosiloxane CAS 9004-73-3View Details
9004-73-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4