POLYETHYLENEIMINE
Synonym(s):Ethyleneimine polymer solution;PEI
- CAS NO.:9002-98-6
- Empirical Formula: C2H5N
- Molecular Weight: 43.07
- MDL number: MFCD00803910
- EINECS: 618-346-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
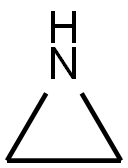
What is POLYETHYLENEIMINE?
Chemical properties
Clear viscous liquid
The Uses of POLYETHYLENEIMINE
Polyethyleneimine is used as a polyelectrolyte multilayer on charged surfaces to provide a biocompatible coating on surfaces.1Detergents, adhesives, water treatment, printing inks, dyes, cosmetics, and paper industry, adhesion promoter, lamination primer, fixative agent, flocculant, cationic dispersant, stability enhancer, surface activator, chelating agent, scavenger for aldehydes and oxides.
The Uses of POLYETHYLENEIMINE
Polyethyleneimine acts as a protein precipitant used to purify proteins. It is used as a chelating agent and as a scavenger for aldehydes and oxides. It is also used in detergents, paper industry, dyes, printing inks and in water treatment.
The Uses of POLYETHYLENEIMINE
Branched polyethyleneimine (PEI) is widely used in many applications due to its polycationic character. Unlike its linear equivalent, branched PEI contains primary, secondary, and tertiary amines. Primarily utilized in industrial applications, high molecular weight PEI has been used as a flocculating agent, textile coating, adhesion promoter, enzyme carrier, and as a material for CO2 capture.
What are the applications of Application
Polyethyleneimine, 50% aqueous solution is a useful protein and nucleic acid precipitation agent
What are the applications of Application
Polyethyleneimine, 30% in water is used for protein and nucleic acid precipitation, transfection assays, and to pretreat filters to increase the binding of proteins
Definition
ChEBI: Aziridine is a saturated organic heteromonocyclic parent, a member of aziridines and an azacycloalkane. It has a role as an alkylating agent. It is a conjugate base of an aziridinium.
Production Methods
Polyethylenimine is produced by the homopolymerization of ethylenimine. The reaction is catalyzed by acids, Lewisacids, or haloalkanes. The polymerization is usually carried out at 90 – 110 ℃ in water or in a variety of organic solvents. The average molecular mass of the polyethylenimine prepared as described above is 10 000 – 20 000. Higher molecular mass polymers are prepared by addition of a difunctional alkylating agent, such as chloromethyloxirane or 1,2-dichloroethane. Polyethylenimines with a higher average molecular mass can also be provided by ultrafiltration of polymers with a broad mass distribution. Likewise, polymers of lower molecular mass can be obtained by inclusion of a low molecular mass amine, such as 1,2- ethanediamine, during polymerization. By using these techniques a range of molecular masses from 300 to 10 6 can be obtained. Cross-linking during the polymerization of ethylenimine in organic solvents leads to solid polyethylenimines. Furthermore the polymerization process can be conducted on the surface of organic or inorganic materials, thus fixing the polyethylenimines to a support.
What are the applications of Application
Polyethyleneimine(PEI) can be used as a non-viral synthetic polymer carrier for in vivo delivery of therapeutic nucleic acids. The interaction between the negatively charged nucleic acid and the positively charged polymer backbone leads to the formation of nanoscale complexes. This neutralising complex protects the enclosed nucleic acid from enzymes and maintains its stability until cellular uptake occurs. For example, human serum albumin-coupled PEI shows good pDNA transfection and low toxicity.
PEI can be used to functionalize single-walled nanotubes (SWNTs) to improve their solubility and biocompatibility while maintaining the structural integrity of the original SWNT. Covalently functionalized SWNTs can be used for CO2 uptake and gene delivery.
Branched PEI can also be used to modify the surface properties of adsorbents. PEI-modified aqueous zirconia/PAN nanofibres have a high fluoride adsorption capacity and a wide working pH range, and can therefore be used for groundwater defluoridation.
General Description
All polyethylene imine polymers are hydrophilic and may contain approx. 30% hydrated water.
Trade name
Lupasol, Polymin, Catiofast, Lugalvan (BASF), Epomin (Nippon Shokubai).
Biological Activity
Polyethylenimine is nondegradable and the molecular weight of PEI affects the cytotoxicity and gene transfer activity. Polyethylenimine acts as a low toxicity and efficient gene vector.
Structure and conformation
Polyethyleneimine(PEI) exists as both a branched and linear structure. Branched PEI (bPEI) is synthesized via acid-catalyzed polymerization of aziridine, whereas the linear structure (lPEI) is synthesized via ring opening polymerization of 2-ethyl-2-oxazoline followed by hydrolysis.
Properties of POLYETHYLENEIMINE
| Melting point: | 59-60°C |
| Boiling point: | 250 °C(lit.) |
| Density | 1.030 g/mL at 25 °C |
| vapor pressure | 9 mmHg ( 20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | DMSO (Sparingly) |
| form | Liquid |
| color | Pale yellow |
| Specific Gravity | 1.045 (20/4℃) |
| PH | pH(50g/l, 25℃) : 10~12 |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| InChI | InChI=1S/C2H5N/c1-2-3-1/h3H,1-2H2 |
| EPA Substance Registry System | Aziridine, homopolymer (9002-98-6) |
Safety information for POLYETHYLENEIMINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for POLYETHYLENEIMINE
| InChIKey | NOWKCMXCCJGMRR-UHFFFAOYSA-N |
| SMILES | C1NC1 |
New Products
1-Amino-1-cyclohexanecarboxylic acid 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione S-Methylisothiosemicarbazide hydroiodide ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate Decanonitrile N,N'-diallyl-1,3-diaminopropanedihydrochloride N-(3-Nitrophenyl)cyclopropanecarboxamide (2-amino-2-phenylethyl)(methyl)amine Methyl-2-acetamidobenzoate methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 4-cyano benzaldehyde 2-hydroxy-5-bromo pyridine 5-Fluoro-2-Oxindole 3,5-Dichloro-2,6-Dimethyl-1h-Pyridin-4-One (9H-fluoren-9-yl)methyl carbonochloridate 2-methyl-5-nitroaniline (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acidRelated products of tetrahydrofuran






You may like
-
 Polyethyleneimine, branched, M.W. 10,000 CAS 9002-98-6View Details
Polyethyleneimine, branched, M.W. 10,000 CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine, branched, M.W. 10,000 CAS 9002-98-6View Details
Polyethyleneimine, branched, M.W. 10,000 CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
Polyethyleneimine on silica beads anion exchange resin CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine, Linear, M.W. 25,000 CAS 9002-98-6View Details
Polyethyleneimine, Linear, M.W. 25,000 CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine, Linear, M.W. 25,000 CAS 9002-98-6View Details
Polyethyleneimine, Linear, M.W. 25,000 CAS 9002-98-6View Details
9002-98-6 -
 Polyethyleneimine, branched, M.W. 600 CAS 9002-98-6View Details
Polyethyleneimine, branched, M.W. 600 CAS 9002-98-6View Details
9002-98-6