Pixantrone
- CAS NO.:144510-96-3
- Empirical Formula: C17H19N5O2
- Molecular Weight: 325.37
- MDL number: MFCD09837694
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
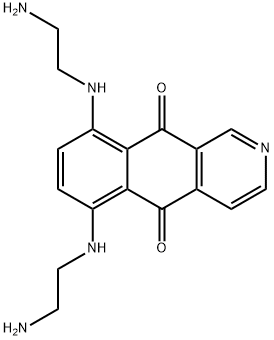
What is Pixantrone?
Absorption
Intravenous administration results in a rapid distribution followed by a slow elimination. [2] In ex vivo myocardial strips, pixantrone is taken up to a higher degree than mitoxantrone. In myocardial strips which are doxorubicin naive pixantrone displays higher uptake than in DOX-loaded myocardial strips. DOX clearance causes membrane effects which may be responsible for this observation. DOX clearance involves rapid passive diffusion through one side of the membrane followed by "flip flop" reorientation of the lipid bilayer. This disorganization of lipids is believed to impair membrane penetration by pixantrone. [3]
Toxicity
Pixantrone appears well tolerated. The most common toxicity is neutropenia. Other toxicities include lymphopenia, thrombocytopenia, alopecia, nausea and vomiting. As pixantrone is a blue compound patients may experience a blue discoloration of the skin and urine. [2]
Description
In May 2012, pixantrone was approved by the European Commission as a single agent for the treatment of relapsed or refractory aggressive B-cell non-Hodgkin lymphoma (NHL) in adult patients who have failed on at least two previous therapies. Pixantrone (also known as BBR- 2778) is an anthracycline analogue that was specifically designed to address the cardiotoxicity seen in earlier agents by replacement of a 1,4- dihydroxyanthracene-9,10-dione core with a benzoisoquinoline-5,10-dione ring system. Pixantrone inhibits topoisomerase II by intercalation with DNA and is also believed to form covalent adducts with the N-2 amino group of guanine via a formaldehyde aminal formed with the primary amino groups. Pixantrone is less cytotoxic than other anthracycline derivatives, but shows good antitumor activity in vivo in a variety of preclinical tumor models, including leukemia and lymphoma models. Pixantrone also demonstrated significantly reduced cardiotoxicity in preclinical models compared with the anthracyclines doxorubicin and mitroxantrone. Pixantrone was synthesized by Friedel–Crafts reaction of pyridine-3,4-dicarboxylic acid anhydride with 1,4-difluorobenzene to give a ketoacid that was cyclized to the tricyclic core by treatment with fuming sulfuric acid at 140℃. Reaction of the resulting 6,9-difluorobenzoisoquinoline-5-10-dione with ethylenediamine followed by careful pH adjustment and treatment with maleic acid gave pixantrone in good overall yield and purity.
Originator
University of Vermont (United States)
The Uses of Pixantrone
Pixantrone is an antineoplastic drug belonging to group of antitumor antibiotics. Pixantrone is an anlogue of Mitoxantrone (M373425) and is just as potent in the treatment of multiple sclerosis with fewer toxic effects on cardiac tissue. Studies suggest that Pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity, a mechanism implicated in Alzheimer's disease.
The Uses of Pixantrone
anti-inflammatory and immunosuppressive glucocorticoid steroid
Background
Pixantrone is an aza-anthracenedione and DNA intercalator which inhibits topoisomerase II. It is similar in structure to anthracyclines such as mitoxantrone, but exerts fewer toxic effects on cardiac tissue. [2] The lower cardio-toxic effects of pixantrone may be explained, in part, by its redox inactivity [3]. Pixantrone does not bind iron and promotes the formation of reactive oxygen species to a lesser degree than other anthracyclines. It also inhibits doxorubicinol formation in human myocardium. [3] As a result, it is believed to be less cardiotoxic while still exerting efficacy.
Pixantrone was designed to treat relapsed or refractory aggressive non-Hodgkin's lymphoma(NHL) in patients who have failed two prior lines of therapy. [2] For patients suffering from NHL, first line therapies consist of anthracycline containing multi-drug treatments which unfortunately are known to cause irreversible myocardial tissue damage. Patients refractory to treatment, or those who relapse, are discouraged from further anthracycline use due to cumulative cardiotoxicity. Pixantrone dimaleate, administered intravenously, was designed by Cell Therapeutics Incorporated as an alternative second line therapy in refractory or relapsed NHL. It is currently being tested in Phase III trials. [2]
Although pixantrone has not yet received FDA approval in the United States, it has been granted conditional marketing approval by the European Union. Conditional approval was granted by the European Medicines Agency after a phase III EXTEND trial of patients with NHL showed that pixantrone was tolerable and that it resulted in significantly higher complete response rate and progression free survival in comparison to other single chemotherapy agents. However, it is notable that the EXTEND trial was stopped early, leaving the statistical significance of the results in question. Based on this uncertainty, in 2009, the FDA ultimately rejected Cell Therapeutic's initial application for accelerated approval for pixantrone use in relapsed or refractory NHL. Another phase III trial, PIX-R, is now ongoing to clarify pixantrones place in therapy. It will compare pixantrone efficacy to that of gemcitabine. [2]
Indications
Currently in Phase III investigation for treatment of relapsed or refractory aggressive non-Hodgkin's lymphoma in patients who have failed two prior lines of therapy. Presently, no standard therapy exists for patients with relapsed or refractory NHL. [2] After first line therapy has been initiated, most patients have received their lifetime limit of doxorubicin and further use of anthracyclines may potentially lead to anthracycline-induced congestive heart failure (CHF). Pixantrone is an attractive alternative as a second line agent, due to its lack of cardiac toxicity. [2]
The phase III trial, PIX-R, is ongoing and will compare pixantrone multidrug therapy with an equivalent regimen in patients with diffuse large B-cell lymphoma (the most common type of NHL).
Previous study results have also suggested the possibility that pixantrone may be safe and effective in doxorubicin naive patients. In myocardial strips which are doxorubicin naive, pixantrone is taken up to a higher degree than in myocardial strips which are doxorubicin exposed, and once absorbed exhibits redox inactivity. [3]
Pixantrone dimaleate has also been investigated as a treatment for acute myelogenous leukemia, diffuse large B-cell lymphoma, follicular lymphoma, metastatic breast cancer, low grade small lymphocytic lymphomas and general metastatic cancers.
Definition
ChEBI: Pixantrone is a member of isoquinolines.
brand name
Pixuvri
Pharmacokinetics
Pixantrone has a wide range of antitumor activity, especially in terms of treating leukemias and lymphomas [3].
Pixantrone lacks cardio-toxic effects. It has postulated that his is because of its redox inactivity and lack and inhibition of doxorubicinol formation in human myocardium. [3]
Clinical Use
Pixuvri (Pixantrone dimaleate) is a novel aza-anthracenedione derivative approved in Europe for the treatment of adult patients with non-Hodgkin B-cell lymphoma. It is also being pursued as a treatment for various cancers, and specifically as an alternative to other structurally-related drugs like mitoxantrone, employed for treatment of breast cancer, acute myeloid leukemia (AML), and non- Hodgkins lymphoma.Pixantrone dimaleate has been designed to maintain antitumor efficacy while decreasing highly cardiotoxic side effects observed during treatment with other related anti-tumor anthracenedione derivatives. Like many anthracenedione drugs, the mechanism of action for pixantrone dimaleate likely includes a number of pathways and processes, with studies suggesting intercalation into DNA and/or interference with DNA –Topoisomerase II activity, leading to subsequent protein associated-DNA strand breaks and eventually to cell death.
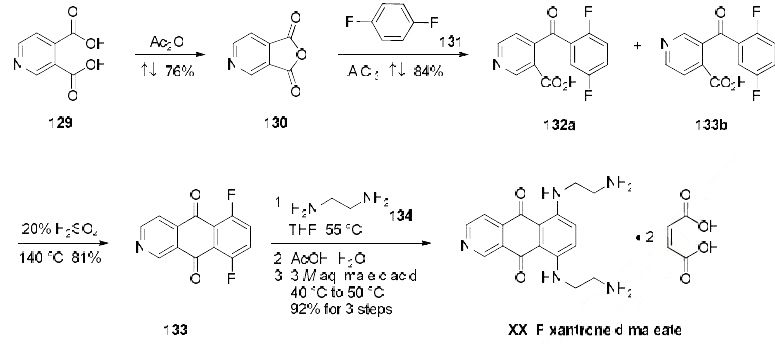
Synthesis
Pixantrone dimaleate, also
known as BBR 2778, was originally synthesized by professors Krapcho and Hacker at the University of
Vermont, and determination of in-vitro tumor cell cytotoxicity was co-identified by the Boehringer
Mannheim Italia research center and the University of Vermont. After the merger of Boehringer
Mannheim with La-Roche, Novuspharm, and Cell Therapeautics, Inc., pixantrone dimaleate has been
developed and marketed by Cell Therapeutics, Inc.
The manufacturing scale synthesis of pixantrone dimaleate relies on several process modifications, from the original synthesis reported by Krapcho in 1994.169 This modified procedure has provided
active pharmaceutical ingredient (API) in high purity (>99%) and is acceptable for use in
pharmaceutical applications (the scheme). Beginning with pyridine 3,4-dicarboxylic acid (129),
generation of the corresponding anhydride 130 proceeded in 76% yield upon treatment with refluxing
Ac2O. Next, an AlCl3-promoted Friedel-Crafts reaction of 1,4-difluorobenzene (131) with 130 under
reflux conditions provided a mixture of nicotinic acid isomers 132a/132b in 84% yield, which were
carried directly to the next step. Cyclization with fuming H2SO4 yielded the desired difluorobenzoisoquinoline-
dione core 133, which was further functionalized with ethylenediamine (134) to provide
the free base of pixantrone. Subjection of the pixantrone free base to aqueous acetic anhydride and
maleic acid provided pixantrone dimaleate (XX) in 92% yield over 3 steps.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Live vaccines: risk of generalised infections - avoid.
Metabolism
Pixantrone may be metabolised in the liver and/or excreted in the bile. As metabolism appears to be limited, biliary excretion of unchanged pixantrone may be the major elimination pathway. Acetylated metabolites were pharmacologically inactive and metabolically stable. In human urine, the compound was mainly excreted unchanged, and very small amounts of phase I and phase II acetylated metabolites were found.
Metabolism
Pixantrone does not form secondary alcohol metabolites. [2] Pixantrone hydrolyzes extensively to CT-45886 which is believed to inhibit doxol formation by displacing DOX from the active site of reductases. CT4889 and CT-45890 are also formed.[3]
Properties of Pixantrone
| Melting point: | >173°C (dec.) |
| Boiling point: | 650.0±55.0 °C(Predicted) |
| Density | 1.405 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 9.34±0.10(Predicted) |
| form | Solid |
| color | Dark Blue to Very Dark Blue |
Safety information for Pixantrone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pixantrone
Pixantrone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![BENZ[G]ISOQUINOLINE-5,10-DIONE](https://img.chemicalbook.in/CAS/GIF/46492-08-4.gif)
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
