Pirprofen
- CAS NO.:31793-07-4
- Empirical Formula: C13H14ClNO2
- Molecular Weight: 251.71
- MDL number: MFCD00866096
- EINECS: 250-805-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
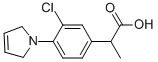
What is Pirprofen?
Originator
Rengasil, Ciba Geigy ,France ,1981
The Uses of Pirprofen
Pirprofen is a non-steroidal anti-inflammatory drug.
The Uses of Pirprofen
Anti-inflammatory.
Definition
ChEBI: Pirprofen is a pyrroline.
Manufacturing Process
To the mixture of 85.5 g ethyl α-(3-chloro-4-aminophenyl)-propionate hydrochloride, 142 g sodium carbonate and 600 ml dimethyl formamide, 107g 1,4-dibromo-2-butene are added dropwise while stirring and the whole is refluxed for 5 hours and allowed to stand overnight at room temperature. The mixture is filtered, the filtrate evaporated in vacuo, the residue is triturated with hexane, the mixture filtered, the residue washed with petroleum ether and the filtrate evaporated. The residue is combined with 280 ml 25% aqueous sodium hydroxide and the mixture refluxed for 8 hours. After cooling, it is diluted with water, washed with diethyl ether, the pH adjusted to 5 to 5.2 with hydrochloric acid and extracted with diethyl ether. The extract is dried, filtered, evaporated and the residue crystallized from benzene-hexane, to yield the α-(3-chloro-4-pyrrolinophenyl)-propionic acid melting at 94°C to 96°C.
Therapeutic Function
Antiinflammatory
World Health Organization (WHO)
Pirprofen, a nonsteroidal anti-inflammatory agent, was introduced in 1982 primarily for the treatment of rheumatic diseases, as well as for use in posttraumatic and post-operative inflammatory conditions, acute gout and dysmenorrhoea. Reports of serious adverse effects, in particular cases of liver toxicity, some of which were fatal, led the manufacturer, in 1985 and in 1989, to amend the approved product information of the drug, limiting duration of treatment and lowering the recommended doses. In the light of these successive restrictions, which have considerably reduced the field of application of pirprofen and in view of available alternatives, the manufacturer has decided to discontinue the drug worldwide.
Trade name
Rengasil (Ciba, Greece), Seflenyl (Geigy, Argentina).
Clinical Use
Pirprofen has been used to treat rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. An optimal dosing regimen of 200 mg three times a day has been developed for maximal activity with minimal adverse effects. Pirprofen also is effective in relieving pain from malignant disease and oral surgery.
Synthesis
Synthesis: treatment of the sodium
salt of diethyl methylmalonate with 2,4-
dichloronitrobenzene yields diethyl (3-chloro-
4-nitrophenyl)methylmalonate. Saponification,
decarboxylation, and subsequent reesterification followed by catalytic reduction gives ethyl
4-amino-3-chloro-α-methylbenzeneacetate hydrochloride. Treatment of the latter with 1,4-
dichloro-2-butene in the presence of sodium
carbonate followed by saponification affords
pirprofen.

Properties of Pirprofen
| Melting point: | 98-100° |
| Boiling point: | 410.8±45.0 °C(Predicted) |
| Density | 1.1778 (rough estimate) |
| refractive index | 1.5270 (estimate) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | 4.56±0.10(Predicted) |
| form | Solid |
| color | Off-White |
Safety information for Pirprofen
Computed Descriptors for Pirprofen
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4