Pirbuterol
- CAS NO.:38677-81-5
- Empirical Formula: C12H20N2O3
- Molecular Weight: 240.3
- MDL number: MFCD00867058
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 16:28:12
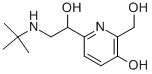
What is Pirbuterol?
Toxicity
As with all sympathomimetic aerosol medication, cardiac arrest and even death may be associated with abuse of pirbuterol.
Description
Pirbuterol is a β2-agonist in the management of disorders with reversible airways obstruction. It is given by inhalation and orally.
Originator
Exirel ,Pfizer Taito ,Japan,1982
The Uses of Pirbuterol
Bronchodilator.
The Uses of Pirbuterol
This relatively selective β2-adrenergic receptor agonist is structurally very similar to albuterol, and it displays similar broncholytic properties. It is used as an inhaled drug for treating bronchial asthma.
Indications
For the prevention and reversal of bronchospasm in patients 12 years of age and older with reversible bronchospasm including asthma.
Background
Pirbuterol is a beta-2 adrenergic bronchodilator. In vitro studies and in vivo pharmacologic studies have demonstrated that pirbuterol has a preferential effect on beta-2 Adrenergic receptors compared with isoproterenol. While it is recognized that beta-2 adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta-2 receptors in the human heart, existing in a concentration between 10-50%. The precise function of these receptors has not been established.
The pharmacologic effects of beta adrenergic agonist drugs, including pirbuterol, are at least in proof attributable to stimulation through beta adrenergic receptors of intracellular adenyl cyclase, the enzyme which catalyzes the conversion of adenosine triphosphate (AlP) to cyclic-3? ,5?-adenosine monophosphate (c-AMP). Increased c-AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Definition
ChEBI: Pirbuterol is a member of pyridines.
Manufacturing Process
To 78 ml of a 1 M solution of diborane in tetrahydrofuran under nitrogen and cooled to 0°C is added dropwise over a period of 40 minutes 13.5 g of N-tertbutyl-2-(5-benzyloxy-6-hydroxymethyl-2-pyridyl)-2-hydroxyacetamide in 250 ml of the same solvent. The reaction mixture is allowed to stir at room temperature for 3.5 hours, and is then heated to reflux for 30 minutes and cooled to room temperature. Hydrogen chloride (70 ml, 1.34 N) in ethanol is added dropwise, followed by the addition of 300 ml of ether. The mixture is allowed to stir for 1 hour and is then filtered, yielding 11.0 g, melting point 202°C (dec.). The hydrochloride dissolved in water is treated with a sodium hydroxide solution to pH 11 and is extracted into chloroform (2 x 250 ml). The chloroform layer is dried over sodium sulfate, concentrated to dryness in vacuo, and the residue recrystallized from isopropyl ether, 3.78 g, melting point 81°C to 83.5°C.
A solution of 1.7 g of 2-hydroxymethyl-3-benzyloxy-(1-hydroxy-2-tert-butylaminoethyl)pyridine in 30 ml of methanol containing 1.2 ml of water is shaken with 700 mg of 5% palladiumon-charcoal in an atmosphere of hydrogen at atmospheric pressure. In 17 minutes the theoretical amount of hydrogen has been consumed and the catalyst is filtered. Concentration of the filtrate under reduced pressure provides 1.4 g of the crude product as an oil. Ethanol (5 ml) is added to the residual oil followed by 6 ml of 1.75 N ethanolic hydrogen chloride solution and, finally, by 5 ml of isopropyl ether. The precipitated product is filtered and washed with isopropyl ether containing 20% ethanol, 1.35 g, melting point 182°C (dec.).
brand name
Maxair (3M Pharmaceuticals).
Therapeutic Function
Bronchodilator
Pharmacokinetics
Pirbuterol is a beta-2 adrenergic bronchodilator. In vitro studies and in vivo pharmacologic studies have demonstrated that pirbuterol has a preferential effect on beta-2 adrenergic receptors compared with isoproterenol. While it is recognized that beta-2 adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta-2 receptors in the human heart, existing in a concentration between 10-50%. The precise function of these receptors has not been established.
Synthesis
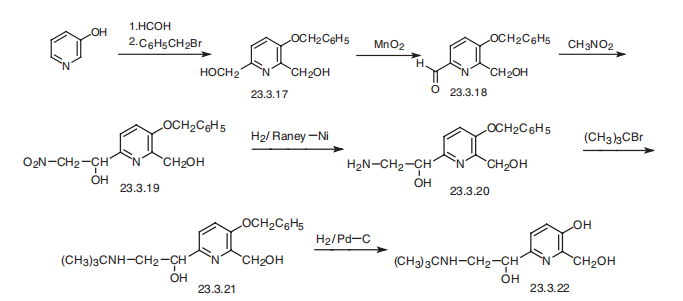
Pirbuterol, |á(6)¨C[[[1,1-dimethylethyl]amino]methyl]-3-hydroxy-2,6-pyridindimethanol (23.3.22), is synthesized from 3-hydroxypyridine, which undergoes subsequent hydroxymethylation and further alkylation by benzylchloride at the aromatic hydroxyl group, giving 3-benzyloxy-2,6-bis-(hydroxymethyl)pyridine (23.3.17). Selective oxidation of the 6-hydroxymethyl group using manganese peroxide gives 3-benzyloxy-2- hydroxymethylpiperidine-6-aldehyde (23.3.18). Condensation of this with nitromethane gives the corresponding nitromethylcarbinol 23.3.19, the nitro group of which is reduced to an amine group by hydrogen using Raney nickel as a catalyst, which forms an aminoalcohol 23.3.20. Alkylation of the aminogroup with tert-butylbromide gives a secondary amine (23.3.21), and removing the protective benzyl group by hydrogen reduction forms pirbuterol (23.3.22) .
Metabolism
Not Available
Properties of Pirbuterol
| Boiling point: | 489.3±40.0 °C(Predicted) |
| Density | 1.196±0.06 g/cm3(Predicted) |
| pka | pKa 8.01/10.64(H2O,t =25±0.05,I=0.010) (Uncertain) |
| CAS DataBase Reference | 38677-81-5 |
Safety information for Pirbuterol
Computed Descriptors for Pirbuterol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
