piprinhydrinate
- CAS NO.:606-90-6
- Empirical Formula: C19H23NO.C7H7ClN4O2
- Molecular Weight: 0
- MDL number: MFCD00792402
- EINECS: 210-128-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 16:37:02
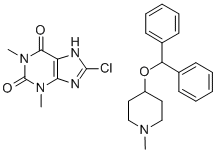
What is piprinhydrinate?
Description
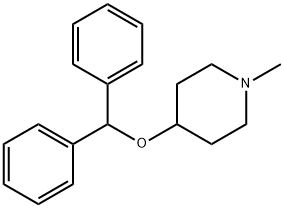
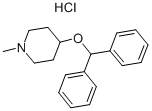
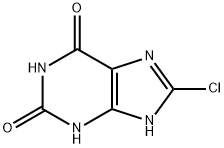
Piprinhydrinate is compositon of Diphenylpyraline and 8-chloroteophylline. 8-Chlorotheophylline is a stimulant drug of the xanthine chemical class, with physiological effects similar to caffeine. Diphenylpyraline is an antihistamine that prevents, but does not reverse, responses mediated by histamine alone. Diphenylpyraline antagonizes most of the pharmacological effects of histamine, including urticaria and pruritus. Also, diphenylpyraline may exhibit anticholinergic actions (as do most of the antihistamines) and may thus provide a drying effect on the nasal mucosa. 8-Chlorotheophylline main use is in combination with Diphenylpyraline or diphenhydramine in the antiemetic Piprinhydrinate and dimenhydrinate. Diphenylpyraline (or diphenhydramine) reduces nausea but causes drowsiness, and the stimulant properties of 8-Chlorotheophylline help ward off that side-effect.
Originator
Piprinhydrinate ,Kraeber and Co. GmbH
Manufacturing Process
A mixture of 46 g of 1-methyl-4-piperidinol (0.4 mol), 49.4 g of benzhydryl bromide (0.2 mol) and 100 ml of xylene was refluxed for approximately 24 hours. The reaction mixture separated into two phases with the upper phase containing the desired ether compound dissolved in xylene. The lower phase consisted of the hydro bromide salt of the excess 1-methyl-4-piperidinol. The upper phase was separated from the lower phase and the desired benzhydryl ether recovered in the crude state by distilling off the xylene under reduced pressure. The crude benzhydryl ether was a clear reddish oil. It was dissolved in 75 ml of 20% hydrochloric acid and the aqueous acid solution then washed three times with 50 ml portions each of ethyl ether. The aqueous acid solution was then decolorized with activated carbon and thereafter slowly admixed with 75 ml of 28% aqueous ammonia. The benzhydryl ether separated as an oily material and was removed from the aqueous mixture by extraction with three 50 ml portions of ethylether. On evaporation of the ethyl ether from the ethyl ether solution, the benzhydryl ether was recovered as a pale yellow oil. The benzhydryl ether was dissolved in 60 ml of isopropanol and the isopropanol solution acidified to a PH of 3 with dry hydrogen chloridemethanol solution. The acidic propanol solution was then diluted with ethyl ether until a faint turbidity was observed. In a short time, the crystalline hydrochloride salt of the benzhydryl ether separated from the propanol solution. The crystallized salt was recrystallized once from 75 ml of isopropanol with the aid of ethyl ether in order to further purify the material. A yield 24.5 g of the pure hydrochloride salt 1-methylpiperldyl-4-benzhydryl ether (diphenylpyraline) was obtained. This was 39% of the theoretical yield. The pure material had a melting point of 206°C.
107 g (0.5 mole) 8-chlorotheophylline was dissolved in the diluted solution of ammonia contained 0.5 moles NH3. 1 equivalent of this ammonim salt was mixed with 1 equivalent hydrochloride of 1-methylpiperldyl-4-benzhydryl ether in 150 ml of water. 4-Diphenylmethoxy-1-methylpiperidine compound of 8chlorotheophylline precipitated, filtered off, washed, dried. Yield was quantitative. MP: 151-152°C.
Therapeutic Function
Antihistaminic, Antiemetic
Properties of piprinhydrinate
| Melting point: | 174-176° |
Safety information for piprinhydrinate
Computed Descriptors for piprinhydrinate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
