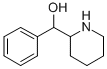
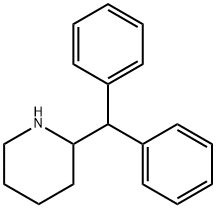
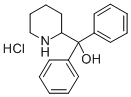
pipradrol
- CAS NO.:467-60-7
- Empirical Formula: C18H21NO
- Molecular Weight: 267.37
- EINECS: 207-394-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
What is pipradrol?
Absorption
Rapidly absorbed .
Toxicity
Toxicity Data: Oral LD50 (rat): 180 mg/kg; Oral LD50 (mouse): 120 mg/kg; Oral LD50 (rabbit): 180 mg/kg .
The toxicity of this drug is dependent on the dose ingested, and is owed to its central nervous stimulant activity , .
Overdoses of pipradrol hydrochloride cause nausea, anxiety, insomnia and abdominal pain, however, these symptoms often disappear when the drug is withdrawn. In severe cases, convulsions may occur , . This drug is contraindicated in anxiety, psychosis states, and schizophrenia, as it can worsen these symptoms. Hallucinations have been reported after taking this drug .
Over the last decade there has been greater use of novel psychoactive substances (‘legal highs’) across Europe and the United States, including increasing frequency of reports of diphenylprolinol (D2PM) and desoxypipradrol (2-DPMP) use .
There are increasing reports that D2PM (desoxy analogue of pipradrol) and 2-DPMP (a pyrrolidine analogue of pipradrol) are used in Europe as drugs of abuse. 2-DPMP has sympathomimetic properties similar to cocaine and, in addition, prolonged and clinically significant neuropsychiatric symptoms have been reported . The binding and activity of D2PM at the dopamine re-uptake transporter, is also similar to cocaine, though it appears that D2PM has less potent biological activity .
Originator
Alertonic ,Adcock Ingram Ltd.
Background
Pipradrol (Meratran) was initially developed in the 1950s as an antidepressant, however, the adverse effects associated with its use and its abuse potential led to its withdrawal and international regulation . Pipradrol was made illegal in many countries in 1970s because of its potential for abuse. It is currently classified under the Misuse of Drugs Act as a Class C substance .
Experimentation with the drug and its derivatives for recreational purposes has led to many cases of acute toxicity and has been linked to three fatalities. The social and in particular acute clinical harms of pipradrol derivatives have led to their control under the Misuse of Drugs Act 1971 in the UK in 2012 .
Interestingly, this drug has been studied for bactericidal properties, however, is not currently, used for this purpose . In addition to this, it has shown favorable effects in postpartum depressive symptoms .
Indications
Used to manage fatigue and depression , , , . Used as an adjunct therapy in the management of obesity .
Definition
ChEBI: Pipradrol is a diarylmethane.
Manufacturing Process
A mixture of 48 g (0.167 mole) of α,α-diphenyl-2-pyridinemethanol hydrochloride (Emraert et al., Ber. 72B, 1188 (1939); 74B, 714 (1940), 160 ml of ethanol, and 3 0.5 g of Adams' platinum catalyst was shaken under an initial hydrogen pressure of 60 pounds. The theoretical amount of hydrogen was absorbed in 5 hours. The reaction mixture was refluxed, diluted with enough water to dissolve all the white solid, and filtered hot from the catalyst. The filtrate was cooled and filtered; yield of 38 g of α,α-diphenyl-alpha-(2piperidyl)methanol white product melting at 308-309°C with decomposition.
A mixture of 3.5 grams (0.013 mole) of the above α,α-diphenyl-alpha-(2piperidyl)methanol, 4 g (0.05 mole) of formaldehyde (37%), and 6 grams (0.1 mole) of formic acid was refluxed for 2 days. The reaction mixture was treated with 1.3 g (0.013 mole) of conc. hydrochloric acid and vacuum distilled on the steam bath. The residue was recrystallized from butanone to give the α,αdiphenyl-alpha-(2-piperidyl)methanol hydrochloride which melted at 228229°C (dec.).
brand name
Meratran (Marion Merrell Dow);Alertonic;Gerodryl;Leptidrol;Meratonic;Metadin;Peratran;Piridrol;Stimolag fortis.
Therapeutic Function
Central stimulant
World Health Organization (WHO)
Pipradrol, a central nervous system stimulant, was introduced in 1955 for use as an anorexic agent. Pipradrol is controlled under Schedule IV of the 1971 Convention on Psychotropic Substances. (Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV), , , 1971)
Pharmacokinetics
Pipradrol (Meratran) is a psychoactive agent and a central nervous system stimulant that has proven useful in the field of psychiatry .
Pipradrol was initially used as an adjunct in the dietary management of obesity as well as for the management of dementia symptoms. Numerous reports have been made on the properties of pipradrol, demonstrating its favorable effects in the treatment of depression and fatigue in addition to a variety of other conditions including narcolepsy, spasmodic torticollis, schizophrenia and in geriatric practice .
Metabolism
Rapidly metabolized, and not found in plasma approximately 4 hours post administration .
Properties of pipradrol
| Melting point: | 81-82 °C(Solv: ethanol (64-17-5)) |
| Boiling point: | 410.55°C (rough estimate) |
| Density | 1.0449 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| pka | pKa 9.71 (Uncertain) |
Safety information for pipradrol
Computed Descriptors for pipradrol
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8