PHOSPHATIDYLETHANOLAMINE
Synonym(s):L -α-Cephalin;1,2-Diacyl-sn-glycero-3-phosphoethanolamine;3-sn-Phosphatidylethanolamine;L-α-phosphatidylethanolamine (E. coli)
- CAS NO.:39382-08-6
- Empirical Formula: C41H78NO8P
- Molecular Weight: 744.033681
- MDL number: MFCD00135635
- EINECS: 254-438-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
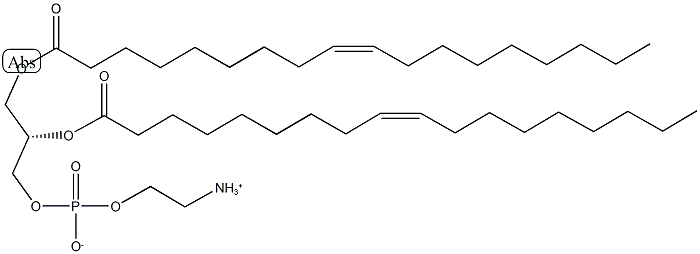
What is PHOSPHATIDYLETHANOLAMINE?
The Uses of PHOSPHATIDYLETHANOLAMINE
L-α-Phosphatidylethanolamine from egg yolk has been used:
- as a component of lipid mixture for the synthesis of giant unilamellar vesicles (GUVs)
- in ceramide phosphoethanolamine (CPE) synthase assay using crude sphingomyelin synthase-related protein from Hela cells
- for the standard curve generation for the quantification of lipids in plantaris muscle homogenates from mice
General Description
L-α-Phosphatidylethanolamine from egg yolk has high unsaturated fatty acid content and elicits radical-scavenging activity. L-α-Phosphatidylethanolamine (PE) is a membrane phospholipid and is interconverted to phosphotidylserine in the presence of phosphatidylserine synthase 2. PE is essential for maintaining the structural integrity of membranes. It is the second most abundant phospholipid in animals, plants and yeast and the major lipid in bacteria. It is synthesized in mitochondria in mammals.
Enzyme inhibitor
This hygroscopic phospholipid, also called cephalin and 1,2-diacyl-snglycero- 3-phosphoethanolamine, is an important constituent of biomembranes and lipoproteins. It is the major structural phospholipid in brain tissue. The physical properties depend on the nature of the acyl groups and on the presence of other lipids. Freshly prepared phosphatidylethanolamines are white solids that turn yellow and brown on exposure to air. They typically sinter between 80 and 90°C and melt in the neighborhood of 175°C. Solutions of phosphatidylethanolamine slowly decompose at room temperature (roughly 0.3-0.5% per day); hence, solutions should always be freshly prepared and kept cold prior to use. It is labile in alkaline conditions. See also specific compound Target(s): b- N-acetylglucosaminyl-glycopeptide b-1,4-galactosyltransferase; Nacetyllactosamine synthase; acylglycerol kinase, or monoacylglycerol kinase; cholesterol monooxygenase, side-chain-cleaving, or CYP11A1; chymase; cytidylate cyclase; dolichyldiphosphatase; dolichyl-phosphatase; dopamine b-monooxygenase; ethanolaminephosphotransferase; glutamate dehydrogenase; γ-glutamyl transpeptidase, mildly inhibited; glycerol-3-phosphate Oacyltransferase; glycerone-phosphate O-acyltransferase, or dihydroxyacetione-phosphate O-acyltransferase; hormone-sensitive lipase; 3-hydroxybutyrate dehydrogenase; indole-3-acetate b-glucosyltransferase; phosphatidate cytidylyltransferase; 1- phosphatidylinositol 4-kinase; phosphoinositide phospholipase C; phospholipase D, phosphatidylcholine-specific enzyme; sphingomyelin phosphodiesterase; steryl-b-glucosidase; UDP-Nacetylglucosamine: dolichyl-phosphate N-acetylglucosaminephosphotransferase.
Purification Methods
Purify the cephalin by dissolving it in EtOH, adding Pb(OAc)2.3H3O (30g in 100mL H2O) until excess Pb2+ is present. Filter off the solid. Pass CO2 gas through the solution until precipitation of PbCO3 ceases. Filter the solid off and evaporate (while bubbling CO2) under vacuum. An equal volume of H2O is added to the residual oil and extracted with hexane. The hexane extract is washed with H2O until the aqueous phase is free from Pb [test with dithizone (2 mg in 100 mL CCl4; Feigel Spot Tests Vol I, Elsevier p. 10 1954)]. The hexane is dried (Na2SO4), filtered and evaporated to give a yellow waxy solid which should be dried to constant weight in vacuo. It is practically insoluble in H2O and Me2CO, but freely soluble in CHCl3 (5%) and Et2O, and slightly soluble in EtOH. [Schofield & Dutton Biochemical Preparations 5 5 1957.]
Properties of PHOSPHATIDYLETHANOLAMINE
| Density | approximate 1.47g/ml(20℃) |
| storage temp. | -20°C |
| solubility | chloroform: 50 mg/mL, slightly hazy, brown-yellow |
| form | solution |
| color | Off-white to light yellow |
Safety information for PHOSPHATIDYLETHANOLAMINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for PHOSPHATIDYLETHANOLAMINE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran






You may like
-
 L-α-Phosphatidylethanolamine from egg yolk CAS 39382-08-6View Details
L-α-Phosphatidylethanolamine from egg yolk CAS 39382-08-6View Details
39382-08-6 -
 L-α-Phosphatidylethanolamine from Glycine max (soybean) CAS 39382-08-6View Details
L-α-Phosphatidylethanolamine from Glycine max (soybean) CAS 39382-08-6View Details
39382-08-6 -
 L-α-Phosphatidylethanolamine from egg yolk CAS 39382-08-6View Details
L-α-Phosphatidylethanolamine from egg yolk CAS 39382-08-6View Details
39382-08-6 -
 Phosphatidylethanolamine CAS 39382-08-6View Details
Phosphatidylethanolamine CAS 39382-08-6View Details
39382-08-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
