PHOSPHAMIDON
Synonym(s):2-Chloro-N,N-diethyl-3-(dimethylphosphono)crotonic amide
- CAS NO.:13171-21-6
- Empirical Formula: C10H19ClNO5P
- Molecular Weight: 299.69
- MDL number: MFCD00055301
- EINECS: 236-116-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
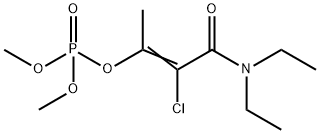
What is PHOSPHAMIDON?
Description
Types of phosphamidon formulations include soluble liquid, suspension concentrate and emulsifiable concentrate, ULV liquid, and 10% granules Phosphamidon is a pale yellow to colourless oily liquid with a faint odour. It is miscible with water and is soluble in aromatic hydrocarbons. Technical phosphamidon is a pale yellow to colourless oily liquid with a faint odour. It consists of a mixture of (Z)-isomer and (E)-isomer in the approximate proportion of 70:30. It decomposes on heating and releases highly toxic fumes such as phosphorus oxides, hydrogen chloride, and nitrogen oxides. Phosphamidon reacts and gets rapidly hydrolysed by alkalis and decomposes on heating or on burning, producing highly toxic fumes. It attacks metals such as iron, tin, and aluminium. It is used as a broad-spectrum insecticide and acaricide for the control of pests and vectors on crops like sugarcane, rice, citrus orchards, and cotton.
Chemical properties
Phosphamidon is a pale yellow to colorless oily liquid with a faint odor. It is miscible with water and is soluble in aromatic hydrocarbons. Phosphamidon decomposes on heating and releases highly toxic fumes, such as phosphorus oxides, hydrogen chloride, and nitrogen oxides. It reacts with bases (hydrolysis) and attacks metals such as iron, tin, and aluminium. Phosphamidon should be handled by trained personnel wearing protective clothing. Phosphamidon is used as a broad-spectrum insecticide and acaricide for the control of pests and vectors on crops like sugar cane, rice, citrus orchards, and cotton. Occupational exposures to phosphamidon occur among factory workers involved in synthesizing formulation and dispensing spray operations. Human exposures also occur among crop harvesters and in vector control operations.
Chemical properties
Phosphamidon is a pale yellow oily liquid
The Uses of PHOSPHAMIDON
Phosphamidon is used to control sucking and boring insects and mites in a very wide range of crops and in forestry applications.
The Uses of PHOSPHAMIDON
Insecticide.
The Uses of PHOSPHAMIDON
Insecticide used to control sap-feeding insects and other pests in a wide variety of crops
Definition
ChEBI: Phosphamidon is a trialkyl phosphate, an organophosphate insecticide, an organochlorine insecticide and an organophosphate nematicide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an EC 3.1.1.8 (cholinesterase) inhibitor, an acaricide and an agrochemical. It is functionally related to a N,N-diethyl-3-hydroxybut-2-enamide.
General Description
Pale yellow oily liquid with a faint odor. Used as an insecticide for citrus, cotton, and deciduous fruit and nuts. and as an acaricide.
Air & Water Reactions
Water soluble. Hydrolyzed by alkali with a half-life at 73°F of 13.8 days at pH 7 and 2.2 days at pH 10 .
Reactivity Profile
PHOSPHAMIDON is corrosive to iron, tin and aluminum. Incompatible with alkaline preparations and should not be mixed with copper oxychloride, captan, folpet or sulfur.
Health Hazard
PHOSPHAMIDON is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150-lb person. It is a cholinesterase inhibitor.
Health Hazard
Phosphamidon is readily absorbed from the gastrointestinal tract, through the intact skin, and by inhalation of spray mists and dusts. Prolonged exposures to phosphamidon cause adverse effects and impairment on the respiratory, myocardial, and neuromuscular transmission in animals and humans. Phosphamidon does not cause delayed peripheral neuropathy in hens. The symptoms of poisoning include, but are not limited to, nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, eye pain, blurred vision, constriction or dilation of the pupils, tears, salivation, sweating, and confusion. Prolonged period of exposures to phosphamidon cause incoordination, slurred speech, loss of refl exes, weakness, fatigue, involuntary muscle contractions, twitching, tremors of the tongue or eyelids, and eventually paralysis of the body extremities and the respiratory muscles, involuntary defecation or urination, psychosis, irregular heart beat, unconsciousness, convulsions, coma, respiratory failure or cardiac arrest leading to death. Phosphamidon has caused clastogenic effects in the bone marrow cells of rats and mice. However, the studies are found to be inadequate to arrive at meaningful conclusions about phosphamidon as a human carcinogen and no data are available. The ADI for phosphamidon has been reported as 0.0005 mg/kg body weight. (ADI: acceptable daily intake is an estimate of the amount of a pesticide, expressed on a body weight basis, which can be ingested daily over a lifetime without appreciable health risk.)
Fire Hazard
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) Container may explode in heat of fire. Heat above 320F may cause decomposition and evolution of highly toxic fumes of phosphorus oxides and chlorides. Hydrolyzes in alkali. Stable in neutral and acid media. Hydrolyzes in alkali.
Potential Exposure
This material is used as an insecticide on citrus, cotton, and deciduous fruit and nuts. It is also an acaricide.
Environmental Fate
Chemical/Physical. Emits toxic fumes of chlorine, phosphorus and nitrogen oxides when heated to decomposition (Sax and Lewis, 1987)
Metabolic pathway
The metabolism of phosphamidon has been reviewed by Geissbuhler et al. (1971) and Beynon et al. (1973). Technical phosphamidon consists of two stereochemical isomers in the E:Z ratio of ca. 3:7. The Z-isomer has the greater insecticidal activity. It is important to note that in the case of phosphamidon the isomer with the phosphate ester function trans to the amide group is assigned the Z configuration due to the priority of chlorine, whereas in the case of mevinphos, monocrotophos and dicrotophos it is assigned the E configuration. In all four compounds, the isomers with this configuration (also referred to as the cis-crotonamide or crotonate structure) have the greater insecticidal activities. It is a systemic insecticide which is rapidly translocated in the plant via the xylem. Phosphamidon is rapidly degraded in the environment, the major routes being via N-de-ethylation and cleavage of the P-O-vinyl function. The resultant N,N-diethyl-2-chloroacetoacetamide or N-ethyl-2-chloroacetoacetamide are then degraded via dechlorination and hydrolysis, ultimately to give acetone, diethylamine and ethylamine. Conjugated metabolites have not been identified.
Metabolism
The major routes of degradation are oxidative dealkylation of the amide group and hydrolysis of the vinyl phosphate ester bond. Dechlorination also occurs. In soils, DT50 is 7–25 d depending upon the soil type.
Shipping
UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Degradation
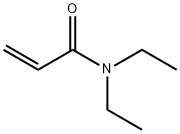
Phosphamidon is rapidly hydrolysed by alkali (PM) but it is relatively stable at neutral and acidic pH values. In alkaline solution, the compound is hydrolysed to N,N-diethyl-2-chloroacetoacetamide (2) and dimethyl phosphate (3) (Anliker and Beringer, 1971). There was apparently no attack on the P-O-Me bond to give the desmethyl compound. In acidic solution compound 2 was hydrolysed to give chloroacetone (4), diethylamine (5) and C02. In alkaline solution 2 was dechlorinated and hydrolysed to acetone (6), acetic acid (7) and glycolic acid diethylamide (8). These pathways are shown in Scheme 1.
Toxicity evaluation
The acute oral LD50 for rats is 17.9–30 mg/kg. Inhalation LC50 (4 h) for rats is about 0.18 mg/L air. NOEL (2 yr) for rats is 1.25 mg/kg b.w. daily. ADI is 0.5 μg/kg b.w. Phosphamidon administered in animals is rapidly metabolized, and 85–90% of the dose is excreted within 24 h almost all in the urine.
Incompatibilities
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Strong oxidizers may cause release of toxic phosphorus oxides. Organophosphates, in the presence of strong reducing agents such as hydrides, may form highly toxic and flammable phosphine gas. Keep away from alkaline materials. Attacks metals, such as aluminum, iron, tin.
Waste Disposal
Small quantities may be treated with alkali followed by landfill disposal. Large quantities should be incinerated with effluent gas scrubbing. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of PHOSPHAMIDON
| Melting point: | 120-123℃ |
| Boiling point: | bp1.5 162°; bp0.001 120° |
| Density | 1.2132 g/cm3 (20 ºC) |
| vapor pressure | 2.2×10-3 Pa (25 °C) |
| refractive index | 1.4718 (589.3 nm 25℃) |
| storage temp. | 2-8°C |
| form | liquid |
| Water Solubility | Totally miscible |
| pka | -1.61±0.70(Predicted) |
| Merck | 13,7423 |
| BRN | 8323501 |
| EPA Substance Registry System | Phosphamidon (13171-21-6) |
Safety information for PHOSPHAMIDON
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for PHOSPHAMIDON
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1