Phosalone
- CAS NO.:2310-17-0
- Empirical Formula: C12H15ClNO4PS2
- Molecular Weight: 367.81
- MDL number: MFCD00055499
- EINECS: 218-996-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
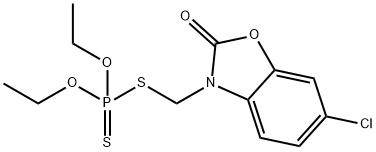
What is Phosalone?
The Uses of Phosalone
Phosalone is a organophosphate compouund used as insecticide and acaricide. Phosalone is a weak inhibitor of acetylcholinesterase.
The Uses of Phosalone
Insecticide, acaricide.
The Uses of Phosalone
Insecticide; acaracide
The Uses of Phosalone
Phosalone is used to control codling moth, aphids, beetles and thrips on fruit trees
Definition
ChEBI: A member of the class of 1,3-benzoxazoles carrying a [(diethoxyphosphorothioyl)sulfanyl]methyl group at the nitrogen atom, an oxo group at position 2 and a chloro group at position 6. It is an organothiophosphate insecticide.
Safety Profile
Poison by ingestion, skin contact,and possibly other routes. A cholinesterase inhibitor. Seealso PARATHION. When heated to decomposition itemits very toxic fumes of Cl-, NOx, POx, and SOx.
Environmental Fate
Plant. Degrades in plants to chlorbenzoxazolone, formaldehyde and diethyl phosphorodithioate (Hartley and Kidd, 1987)
Soil. Ambrosi et al. (1977a) studied the persistence and metabolism of phosalone in
both moist and flooded Matapeake loam and Monmouth fine sandy loam. Phosalone rapidly
degraded (half-life 3 to 7 days) but mineralization to carbon dioxide accoun
Chemical/Physical. Emits toxic fumes of chlorine, phosphorus, nitrogen and sulfur
oxides when heated to decomposition (Sax and Lewis, 1987)
Metabolic pathway
Photodegradation of phosalone in solvent yields dechlorinated phosalone. Irradiation in methanol affords O,O-diethyl-S-methylphosphorothioate and 6- chloro-3-methoxymethyl-2-oxobenzoxazoline with other common products. Photolysis of phosalone in the solid state as a thin film on a glass surface produces a number of photoproducts including 6-chloro-3- mercaptomethyl-2-oxobenzoxazoline and its dimeric disulfide.
Metabolism
Orally administered phosalone in mammals is rapidly degraded by oxidation and hydrolysis to give O,O-diethyl hydrogen phosphorothioate and phosphorodithioate and 6-chloro-2,3-dihydro-2-oxobenzoxazole, which is further metabolized and excreted in the urine. It is strongly adsorbed to soil and rapidly degraded with DT50 values of 1–4 d.
Degradation
Phosalone is hydrolysed by strong acids and alkalis with a DT50, of 9 days at pH 9 (PM).
Properties of Phosalone
| Melting point: | 47.5-48° |
| Boiling point: | 446.7±55.0 °C(Predicted) |
| Density | 1.39 g/cm3 |
| vapor pressure | <6×10-5 Pa (25 °C) |
| Flash point: | 100 °C |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | -1.75±0.20(Predicted) |
| form | solid |
| Water Solubility | 3.05 mg l-1 (25°C) |
| color | Crystals |
| Odor | garlic odor |
| BRN | 694650 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 2310-17-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosalone(2310-17-0) |
| EPA Substance Registry System | Phosalone (2310-17-0) |
Safety information for Phosalone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H332:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Phosalone
| InChIKey | IOUNQDKNJZEDEP-UHFFFAOYSA-N |
Phosalone manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran







You may like
-
 2310-17-0 Phosalone 98%View Details
2310-17-0 Phosalone 98%View Details
2310-17-0 -
 2310-17-0 98%View Details
2310-17-0 98%View Details
2310-17-0 -
 Phosalone CAS 2310-17-0View Details
Phosalone CAS 2310-17-0View Details
2310-17-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1