phenindamine
- CAS NO.:82-88-2
- Empirical Formula: C19H19N
- Molecular Weight: 261.36
- MDL number: MFCD00865675
- EINECS: 201-443-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
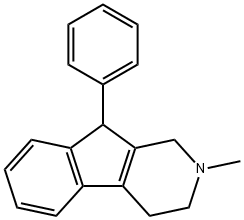
What is phenindamine?
Toxicity
Symptoms of a phenindamine overdose include extreme sleepiness, confusion, weakness, ringing in the ears, blurred vision, large pupils, dry mouth, flushing, fever, shaking, insomnia, hallucinations, and possibly seizures.
Originator
Thephorin ,Roche, US,1947
Background
Phenindamine is an antihistamine. Phenindamine blocks the effects of the naturally occurring chemical histamine in your body. Antihistamines such as phenindamine appear to compete with histamine for histamine H1- receptor sites on effector cells. The antihistamines antagonize those pharmacological effects of histamine which are mediated through activation of H1- receptor sites and thereby reduce the intensity of allergic reactions and tissue injury response involving histamine release. It is used to treat sneezing, runny nose, itching, watery eyes, hives, rashes, itching, and other symptoms of allergies and the common cold. Symptoms of a phenindamine overdose include extreme sleepiness, confusion, weakness, ringing in the ears, blurred vision, large pupils, dry mouth, flushing, fever, shaking, insomnia, hallucinations, and possibly seizures.
Indications
Used to treat sneezing, runny nose, itching, watery eyes, hives, rashes, itching, and other symptoms of allergies and the common cold.
Definition
ChEBI: Phenindamine is an indene.
Manufacturing Process
A mixture of 750 grams of 1-methyl-3-benzoyl-4-hydroxy-4-phenylpiperidine and 2,500 cc of 48% hydrobromic acid is refluxed for about 20 minutes. It is then poured into 8 liters of water. An oily precipitate appears which on standing crystallizes. It is filtered and crystallized from about 3.5 liters of alcohol. 2-Methyl-9-phenyl-2,3-dihydrel-pyridindene hydrobromide, MP 201°203°C, is obtained.
A mixture of 680 grams of 2-methyl-9-phenyl-2,3-dihydrol-pyridindene hydrobromide, 6,000 cc of water and about 100 grams of Raney-nickel catalyst is hydrogenated at room temperature and at about 1,000 lb pressure for a period of three hours. The catalyst is filtered. The clear filtrate is treated with a solution of 240 grams potassium thiocyanate in 400 cc of water. A heavy solid precipitates from which the supernatant liquid is decanted.
The residue is dissolved in 10 liters of boiling alcohol with stirring in the presence of nitro gen. The solution is cooled to room temperature under nitrogen, and then allowed to stand overnight. 2-Methyl-9-phenyl-tetrahydro1-pyridindene thiocyanate separates in crystals of MP 188°-189°C. From the concentrated filtrate an additional amount is obtained. The corresponding free base, prepared by treating the slightly soluble thiocyanate in aqueous suspension with sodium hydroxide and extracting with ether, has a MP of 90°91°C. It forms a tartrate of MP 160°C.
The starting material was prepared by reacting acetophenone, methylamine and formaldehyde followed by treatment of the intermediate with sodium hydroxide.
brand name
Thephorin (Hoffmann-LaRoche).
Therapeutic Function
Antihistaminic
Pharmacokinetics
Phenindamine is an antihistamine. Phenindamine blocks the effects of the naturally occurring chemical histamine in your body. Allergies are caused by an excessive type 1 hypersensitivity response of the body to allergens, mediated by inappropriate histamine signalling. By inhibiting the binding of histamine, antihistamines decrease the normal histamine response from cells, consequently decreasing allergic symptoms.
Metabolism
Not Available
Properties of phenindamine
| Melting point: | 90-91° |
| Density | 1.17 |
| pka | pKa 8.11±0.08(H2O,t undefined,I=0.30(NaCl)) (Uncertain) |
Safety information for phenindamine
Computed Descriptors for phenindamine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4