Phenethyl isothiocyanate
Synonym(s):2-Phenylethyl isothiocyanate;PEITC;Phenethyl isothiocyanate
- CAS NO.:2257-09-2
- Empirical Formula: C9H9NS
- Molecular Weight: 163.24
- MDL number: MFCD00004821
- EINECS: 218-855-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
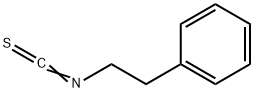
What is Phenethyl isothiocyanate?
Description
Phenethyl isothiocyanate is a naturally occurring isothiocyanate in cruciferous vegetables. Phenethyl isothiocyanate is a chemopreventive agent. It inhibits cancer cell growth by arresting the cell cycle and inducing apoptosis in various human cancer cell models.
Chemical properties
Light Yellow Liquid
Chemical properties
Phenethyl isothiocyanate has a strong green irritating odor.
The Uses of Phenethyl isothiocyanate
Flavor ingredient; provides the hot or burning sensation in horseradish.
Phenethyl isothiocyanate (PEITC),one of the major compounds from dietary cruciferousvegetables,has been found to have antitumor properties and therefore could generate special interest for the development of chemopreventive andor chemotherapeutic agent for human cancers.
The Uses of Phenethyl isothiocyanate
Phenethyl isothiocyanate is an NNK-induced lung tumorigenesis inhibitor.
What are the applications of Application
Phenethyl isothiocyanate is a NNK-induced lung tumorigenesis inhibitor
Definition
ChEBI: An isothiocyanate having a phenethyl group attached to the nitrogen. It is a naturally occurring compound found in some cruciferous vegetables (e.g. watercress) and is known to possess anticancer properties.
Preparation
Prepared by hydrolysis of gluconasturtiin, an abundant natural product present in cruciferous vegetables.
General Description
Phenethyl isothiocyanate, an organic isothiocyanate, is a cruciferous vegetable ingredient present as a glucosinolate in cabbage, turnips, radishes, etc. It is a well-known chemopreventive agent reported to be effective against cancers affecting the lung, breast, pancreas, prostate and so on.
Pharmacokinetics
Phenethyl isothiocyanate (PEITC) is one of the most extensively studied members of the isothiocyanate family, found in cruciferous vegetables, such as watercress. It is responsible for anti-cancer activities. In addition, it has shown to inhibit migration and invasion of many types of human cancer cells. PEITC selectively kills cancer cells, but not normal cells. It generates reactive oxygen species (ROS) to trigger signal transduction, leading to cell cycle arrest, autophagy, and/or apoptosis. However, the molecular mechanisms by which PEITC acts as a growth inhibitor and autophagy and/or apoptosis inducer in cancer cells have not been fully investigated.
PEITC also is able to activate ERK and JNK signal transduction, which in turn induces expression of stress-responsive genes. Specifically, this agent has been shown to reactivate gene expression of a detoxification enzyme, glutathione S-transferase that is silenced in prostate carcinoma.
Phenethyl Isothiocyanate has been used in trials studying the prevention and treatment of Leukemia, Lung Cancer, Tobacco Use Disorder, and Lymphoproliferative Disorders.
Anticancer Research
PEITC is a naturally occurring isothiocyanate, found mostly in cruciferous vegetablesincluding cauliflower, watercress, cabbage, Brussels sprouts, garden cress, broccoli,and bok choy. It shows chemopreventive and anticancer activity againstmelanoma, breast cancer, myeloma, human liver hepatoma, non-small cell lung cancer,cervical cancer, prostate cancer, and osteogenic sarcoma. It induces apoptosis in some drug resistance cell lines. Its mechanism of action is much diversified in variouscancer cells. It induces apoptosis in highly metastatic human non-small cell lungcancer, L9981 cells via caspase-3 activation, and cell cycle arrest in G2/M phase bymodulation of cyclin B1 expression while targeting MAPK/AP-1 pathway. In cervicalcancer cells, it increases the death receptor (DR4, DR5) expression, cleaves caspase-3, induces caspase-8, truncates BID, and downregulates ERK1/2 and MEKphosphorylation while maintaining the expression of JNK and phospho-p38MAPK. In human prostate cancer (DU 145) cell line, PEITC induces apoptosis byactivating caspase cascade pathway (Wang et al. 2012; Cheung and Kong 2010).
Properties of Phenethyl isothiocyanate
| Melting point: | 143~145℃ |
| Boiling point: | 75 °C0.25 mm Hg |
| Density | 1.094 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 4014 | PHENETHYL ISOTHIOCYANATE |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | DMF: 30 mg/ml,DMSO: 30 mg/ml,Ethanol: 30 mg/ml |
| form | Liquid |
| color | Clear colorless to yellow |
| Specific Gravity | 1.09 |
| Odor | at 0.01 % in propylene glycol. green horseradish gooseberry watercress |
| Water Solubility | insoluble |
| Sensitive | Moisture Sensitive |
| Merck | 14,7226 |
| JECFA Number | 1563 |
| BRN | 2084162 |
| CAS DataBase Reference | 2257-09-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, (2-isothiocyanatoethyl)-(2257-09-2) |
| EPA Substance Registry System | Benzene, (2-isothiocyanatoethyl)- (2257-09-2) |
Safety information for Phenethyl isothiocyanate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phenethyl isothiocyanate
| InChIKey | IZJDOKYDEWTZSO-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2-Phenylethyl Isothiocyanate CAS 2257-09-2View Details
2-Phenylethyl Isothiocyanate CAS 2257-09-2View Details
2257-09-2 -
 Phenethyl isothiocyanate CAS 2257-09-2View Details
Phenethyl isothiocyanate CAS 2257-09-2View Details
2257-09-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1