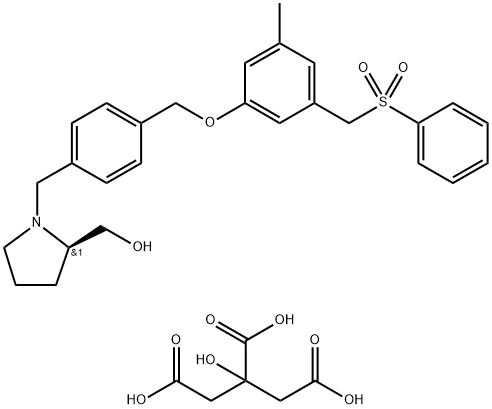
PF 543
Synonym(s):SK Inhibitor II, ( R)-(1-(4-((3-Methyl-5-(phenylsulfonylmethyl)phenoxy)methyl)benzyl)-pyrrolidin-2-yl)methanol, Sphingosine Kinase Inhibitor II;SK Inhibitor II, (R)-(1-(4-((3-Methyl-5-(phenylsulfonylmethyl)phenoxy)methyl)benzyl)-pyrrolidin-2-yl)methanol, Sphingosine Kinase Inhibitor II;Sphingosine Kinase 1 Inhibitor II, PF-543 - CAS 1415562-82-1 - Calbiochem
- CAS NO.:1415562-82-1
- Empirical Formula: C27H31NO4S
- Molecular Weight: 465.6
- MDL number: MFCD23098794
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:30:59
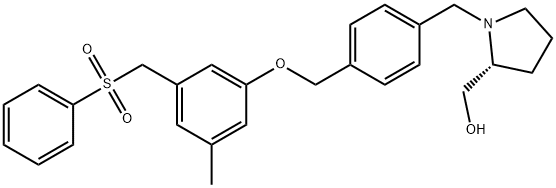
What is PF 543?
Definition
ChEBI: [(2R)-1-[[4-[[3-(benzenesulfonylmethyl)-5-methylphenoxy]methyl]phenyl]methyl]-2-pyrrolidinyl]methanol is a sulfonamide.
General Description
A cell-permeable hydroxymethylpyrrolidine compound that acts as a high affinity (Kd = 5 nM, kofft1/2 = 8.5 min), but poor, sphingosine kinase-1/SphK1 substrate (1/1,500 of sphingosine processivity) and potently inhibits against SphK1-catalyzed sphingosine-to-S1P conversion (IC50 = 2.0 nM; [sphingosine] = 3 μM) in a reversible, sphingosine-, but not ATP-, competitive manner (Ki = 3.6 nM), exhibiting 132-fold selectivity over SphK2, no affinity toward S1P receptors (S1P1, S1P2, S1P3, S1P5), and much reduced or no inhibitory activity against a panel of 46 other lipid and protein kinases (IC50 >10 μM), including the structurally related diacylglyerol kinase α/DAGKα (3.7% inhibition at 10 μM). PF-543 exposure in 1483 squamous cultures is reported to result in intracellular S1P depletion (by 90% upon 1 h 200 nM or 7 d 1 μM drug exposure) with concomitant elevation of sphingosine level and no detectable cytotoxicity (1 μM for 7 to 9 d in 1483, A549, Jurkat, LN229, MCF7, or U937 cultures). Also active in human whole blood assays in the presence human & bovine serum proteins (IC50 = 26.7 nM against cellular C17-S1P formation from exogenously added 20 μM C17-sphingosine in 10 min with 30 min drug preincubation).
Biological Activity
pf-543 is a novel and cell-permeant inhibitor of sphingosine kinase (sphk1) with ic50 value of 3.6 nm [1],sphk1 is a kinase that phosphorylates sphingosine to sphingosine-1-phosphate (sip). sphk1 is normally a cytosolic protein but can be recruited to the membrane to conduct its activity. it is the major source of production of s1p which promotes cell growth, survival and migration, and also regulate lymphocyte trafficking.biochemical study has identified that sphk1 was a sphingosine-competitive inhibitor but not atp-competitive [1]. in 1483 head and neck carcinoma cell cultures expressing high levels of sphk1 and with an unusually high rate of s1p production, pretreatment of pf-543 for 1 hr decreased the level of endogenous s1p by 10-fold with a proportional increase in the level of sphingosine. it indicated a significant inhibition of sphk1 by pf-543. however, specific inhibition of sphk1 had no effect on the proliferation and survival of 1483 cell cultures, despite a dramatic change in the cellular s1p/sphingosine rate [1].the inhibitory activity of pf-543 was also examined ex vivo in human whole blood. human whole blood with high sphk1 activity was prepared, which was able to quickly convert c17-sphingosine to c17-s1p. pf-543 treatment showed that pf-543 was a potent inhibitor of sphk1, capable of blocking >90% c17-s1p formation [1].
Biochem/physiol Actions
PF-543 hydrochloride is a novel, cell-permeable, potent and selective inhibitor of sphingosine kinase-1 (SphK1). PF-543 inhibits SphK1 with a Ki of 3.6 nM, is sphingosine competitive and is more than 100-fold selective for SphK1 over the SphK2 isoform. PF-543 decreased the level of endogenous S1P by 10-fold with proportional increase of the level of sphingosine.
References
[1] schnute m e et al. , modulation of cellular s1p levels with a novel, potent and specific inhibitor of sphingosine kinase-1. biochem j. 2012, 444(1): 79-88.
Properties of PF 543
| Boiling point: | 666.0±55.0 °C(Predicted) |
| Density | 1.224±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | ≥23.3 mg/mL in DMSO; insoluble in H2O; ≥51 mg/mL in EtOH with gentle warming and ultrasonic |
| pka | 14.77±0.10(Predicted) |
| form | powder |
| color | white to light brown |
Safety information for PF 543
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PF 543
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Sphingosine Kinase 1 Inhibitor II, PF-543 CAS 1415562-82-1View Details
Sphingosine Kinase 1 Inhibitor II, PF-543 CAS 1415562-82-1View Details
1415562-82-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4