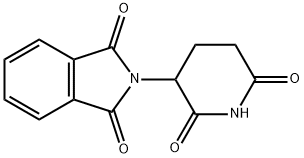
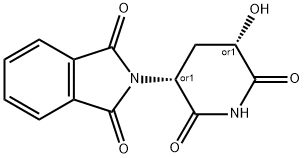
Thalidomide
Synonym(s):(±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;(±)-Thalidomide - CAS 50-35-1 - Calbiochem;(±)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione;(±)-Thalidomide
- CAS NO.:50-35-1
- Empirical Formula: C13H10N2O4
- Molecular Weight: 258.23
- MDL number: MFCD00153873
- EINECS: 200-031-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
What is Thalidomide?
Absorption
The absolute bioavailability has not yet been characterized in human subjects due to its poor aqueous solubility. The mean time to peak plasma concentrations (Tmax) ranged from 2.9 to 5.7 hours following a single dose from 50 to 400 mg. Patients with Hansen’s disease may have an increased bioavailability of thalidomide, although the clinical significance of this is unknown.
Due to its low aqueous solubility and thus low dissolution is the gastrointestinal tract, thalidomide's absorption is slow, with a tlag of 20-40 min. Therefore, thalidomide exhibits absorption rate-limited pharmacokinetics or "flip-flop" phenomenon. Following a single dose of 200 mg in healthy male subjects, cmax and AUC∞ were calculated to be 2.00 ± 0.55 mg/L and 19.80 ± 3.61 mg*h/mL respectively.
Toxicity
The oral LD50 in rats is 113 mg/kg and 2 g/kg in mouse.
Two-year carcinogenicity studies were conducted in male and female rats and mice. No compound-related tumorigenic effects were observed at the highest dose levels of 3,000 mg/kg/day to male and female mice (38-fold greater than the highest recommended daily human dose of 400 mg based upon body surface area [BSA]), 3,000 mg/kg/day to female rats (75-fold the maximum human dose based upon BSA), and 300 mg/kg/day to male rats (7.5-fold the maximum human dose based upon BSA).
Thalidomide was neither mutagenic nor genotoxic in the following assays: the Ames bacterial (S. typhimurium and E. coli) reverse mutation assay, a Chinese hamster ovary cell (AS52/XPRT) forward mutation assay, and an in vivo mouse micronucleus test.
Fertility studies were conducted in male and female rabbits; no compound-related effects in mating and fertility indices were observed at any oral thalidomide dose level including the highest of 100 mg/kg/day to female rabbits and 500 mg/kg/day to male rabbits (approximately 5- and 25- fold the maximum human dose, respectively, based upon BSA). Testicular pathological and histopathological effects (classified as slight) were seen in male rabbits at dose levels ≥30 mg/kg/day (approximately 1.5-fold the maximum human dose based upon BSA).
There is no specific antidote for a thalidomide overdose. In the event of an overdose, the patient’s vital signs should be monitored and appropriate supportive care given to maintain blood pressure and respiratory status.
Description
Thalidomide is a glutamic acid derivative first synthesized in
1953 by Swiss Pharmaceuticals; however, due to lack of pharmacological
effects, the development was discontinued. In
1954, Chemie Grünenthal, a German company, undertook the
development of thalidomide and, in 1957, thalidomide was
marketed as an anticonvulsant for the treatment of epilepsy.
Since the drug caused drowsiness, it was also marketed as
a sedative. Thalidomide was considered a safe and effective
drug that caused deep sleep with no hangover, and by the end
of the 1950s, 14 pharmaceutical firms were marketing the drug
in countries of Europe, Asia, Australia, the Americas, and Africa.
However, the drug was never approved for use in the United
States due to concerns about the safety of the drug raised by
Frances Kelsey, MD, a drug reviewer at Food and Drug
Administration (FDA). The approval process was delayed due
to her repeated requests for additional safety information from
William S. Merrell Company, the licensee of Chemie Grünenthal
that applied to market thalidomide in the United States.
Dr Kelsey’s concerns were mostly related to thalidomideinduced
neuropathy. Previous research had shown that drugs
that irritated nerves in adult rabbits could have adverse effects
on growth and cause deformities in fetal rabbits. During this
time, the use of the drug became widespread and, because it
was effective in alleviating morning sickness, it became popular
among pregnant women. In 1961, two physicians, William G.
McBride, MD of Australia and Widulind Lenz, MD of Germany,
associated the increase in malformation of the limbs (phocomelia)
and other congenital abnormalities with the use of
thalidomide by pregnant women. By late 1961, birth defects in
more than 12 000 children were associated with thalidomide
use, which forced companies to withdraw the drug worldwide.
The birth defects were due to thalidomide teratogenecity:
mainly phocomelia and malformation of ears, often accompanied
by malformation of the internal organ. In 1965, an
experimental use of thalidomide in patients with lepromatous
leprosy proved to be effective in treating painful skin lesions
that resulted from the inflammatory complications of leprosy.
In fact, experimental use of thalidomide had been extended to
a variety of diseases with various degrees of success, including
refractory rheumatoid arthritis, Crohn’s disease, human
immunodeficiency virus (HIV)-1 associated Kaposi’s sarcoma,
cutaneous lupus, prostate cancer, and colorectal cancer. In
1998, the FDA approved Thalomid as a therapy for erythema
nodosum leprosum (ENL), or leprosy. Subsequently in 2006,
Thalomid in combination with dexamethasone was approved
for treatment of multiple myeloma.
Description
Thalidomide has a tragic history: It was introduced in Germany in 1957 as a sedative and hypnotic and was marketed over the counter largely as a drug for treating morning sickness in pregnant women. In the following few years, about 10,000 infants worldwide were born with phocomelia, or limb malformation. Only half of the infants survived, and some of those who did had other defects in addition to limb deficiencies. The thalidomide disaster caused many countries to tighten drug approval regulations.
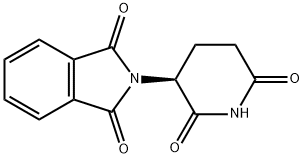
Thalidomide exists in two mirror-image forms: it is a racemic mixture of (R)- and (S)-enantiomers. The (R)-enantiomer, shown in the figure, has sedative effects, whereas the (S)-isomer is teratogenic. Under biological conditions, the isomers interconvert, so separating the isomers before use is ineffective.
More recently, thalidomide has proven useful for treating cancer and leprosy and is approved for these uses. But although more than 2000 papers have been written about its mechanism of teratogenic action, it was not until the past few years that this mechanism was established. In 2010, H. Handa and colleagues at the Tokyo Institute of Technology showed that its biological target is cereblon, a component of an E3 ubiquitin ligase complex. Earlier this year, N. H.?Thom?? and co-workers at the Friedrich Miescher Institute for Biomedical Research (Basel, Switzerland) determined the crystal structure of thalidomide bound to cereblon, which allowed them to characterize the mechanism.
Thalidomide was also the Molecule of the Week for May 17, 2010.
?
Description
(±)-Thalidomide is an immunomodulatory compound with diverse biological activities, including anticancer, anti-inflammatory, and teratogenic properties. It prevents polymorphonuclear leukocyte (PMN) chemotaxis when used at concentrations of 1, 10, and 100 μg/ml. (±)-Thalidomide increases IL-2-induced proliferation and IFN-γ production in primary human T cells in vitro. It enhances natural killer (NK) cell-mediated cytotoxicity in MM.1S multiple myeloma cells. Thalidomide (4 mg/animal) reduces lung IL-6, TGF-β, VEGF, angiopoietin-1, angiopoietin-2, and collagen type Iα1 expression, inhibits pulmonary angiogenesis, and attenuates fibrosis in a mouse model of bleomycin-induced pulmonary fibrosis. It induces apoptosis in primary human embryonic fibroblasts (EC50 = 8.9 μM) and induces limb and eye defects in chicken embryos (EC50 = 50 μg/kg egg weight). Formulations containing thalidomide have been used in the treatment of multiple myeloma and erythema nodosum leprosum (ENL) in non-pregnant individuals.
Chemical properties
White Powder
Originator
Contergan,Grunenthal,Germany
The Uses of Thalidomide
Inhibits FGF-induced angiogenesis. Inhibits replication of human immunodeficiency virus type 1. Teratogenic sedative. There is now a growing clinical interest in Thalidomide, and it is introduced as an immunomodulatory agent used primarily in combination with dexamethasone to treat multiple myeloma.
The Uses of Thalidomide
antiinfective (topical)
The Uses of Thalidomide
Thalidomide was prescribed as an anti-nausea agent to help pregnant women with morning sickness in the late 1950s. It was found to be a potent teratogen, causing many different forms of birth defects and was withdrawn from the market. Thalidomide and synthetic analogs have recently been proven effective in treating inflammation associated with diseases such as leprosy, arthritis and Crohn’s disease, and in cancers such as multiple myeloma. The direct target for the teratogenicity of thalidomide was not discovered until 2010, when it was found that it interacts directly with the protein cereblon (CRBN; IC50 = 8.5 nM), a ubiquitously-expressed E3 ligase. Binding of thalidomide analogs to CRBN-DNA damage binding protein-1 complexes account for the immunomodulatory and antiproliferative effects of these compounds.[Cayman Chemical]
The Uses of Thalidomide
Thalidomide was formerly used as a sedative-hypnotic drug. It is used in the treatmentof leprosy. Recent studies indicate that thecompound may be effective against cancer.
What are the applications of Application
Thalidomide is a selective inhibitor of TNF alpha biosynthesis shown to have immunomodulatory and antiangeogenic activity
Background
A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic, thalidomide was withdrawn from the market due to teratogenic effects. It has been reintroduced and used for a number of inflammatory disorders and cancers. Thalidomide displays immunosuppressive and anti-angiogenic activity through modulating the release of inflammatory mediators like tumor necrosis factor-alpha (TNF-a) and other cytokine action. Due to severe teratogenicity, pregnancy must be excluded before the start of treatment and patients must enrol in the THALIDOMID Risk Evaluation and Mitigation Strategy (REMS) program to ensure contraception adherence.
Indications
Thalidomide is primarily used for the acute treatment and maintenance therapy to prevent and suppress the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL).
Definition
ChEBI: A dicarboximide that is isoindole-1,3(2H)-dione in which the hydrogen attached to the nitrogen is substituted by a 2,6-dioxopiperidin-3-yl group.
Indications
Thalidomide (Thalomid) is a derivative of glutamic acid that is chemically related to glutethimide. It exerts a number of biological effects as an immunosuppressive, antiinflammatory, and antiangiogenic agent, yet its mechanisms of action have not been fully elucidated. Thalidomide potently inhibits production of tumor necrosis factor (TNF) and interleukin (IL) 12, and its effect on these and other cytokines may account for some of its clinical effects.
Manufacturing Process
26 g of N-phthalyl glutaminic acid anhydride are melted with 12 g of urea in an oil bath at 170-180°C until the reaction is completed, which takes about 20 min. The reaction takes place with violent evolution of carbon dioxide and ammonia. After cooling, the reaction product is recrystallised by fractionation from 95% alcohol, and the first fraction may contain phthalic acid derivatives. The required product N-(2,6-dioxo-3-piperidyl)-phthalimide melts at 269- 271°C. The yield is about 65-70% of the theoretical.
brand name
Algosediv;Asidon;Bonbrain;Contergan;Distaval;Funed;Glutanon;Hippuzon;Imidan;Isomin;Kevadone;Nerufatin;Neurosedyn;Pantosedive;Pro-ban;Quetimid;Sanodormin;Sedalis;Sedoval;Shinaito;Shinnibrol;Sleepan;Softenil;Softenon;Talimol;Tlargan;Yodomin.
Therapeutic Function
Sedative, Hypnotic, Antiarthritic
World Health Organization (WHO)
Notwithstanding the highly potent teratogenic action of thalidomide, this drug retains a place in the treatment of reactional lepromatous leprosy and several serious dermatological conditions refractory to other treatment. In many countries, the competent authorities have granted exemption from licensing requirements to enable doctors to obtain limited supplies of thalidomide under strictly controlled circumstances for use in named patients. Arrangements have also been made by some national drug regulatory authorities for thalidomide to be used in institutions concerned with the treatment of leprosy.
Synthesis Reference(s)
Synthetic Communications, 33, p. 1375, 2003 DOI: 10.1081/SCC-120018698
General Description
Needles or white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Organic amides/imides, such as Thalidomide, react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
Thalidomide is a strong teratogen. Exposureto this compound during the first trimesterof pregnancy resulted in deformities inbabies. Infants born suffered from ameliaor phocomelia, the absence or severe shortening of limbs. Administration of thalidomide in experimental animals caused fetaldeaths, postimplantation mortality, and specific developmental abnormalities in theeyes, ear, central nervous system, musculoskeletal system, and cardiovascular system. Several thousand children were affected.The drug has been withdrawn from themarket.Thalidomide is usually administeredorally. Its toxicity is dose dependent. Someother effects are drowsiness, constipation andrash and nerve damage in the arms and legs.Interest in thalidomide resurged in recentyears because of its antitumor activity in thetreatment of multiple myeloma (Oxberry andJohnson 2006). The compound is an inhibitorof angiogenesis, that is, it prevents formationof new blood vessels in tumors. Also, it hasbeen found to be effective in treating AIDS-related Kaposis sarcoma.
Fire Hazard
Flash point data for Thalidomide are not available; however, Thalidomide is probably combustible.
Biological Activity
Teratogen, sedative-hypnotic with inherent anti-inflammatory properties. A selective inhibitor of tumor necrosis factor α (TNF- α ) synthesis.
Biochem/physiol Actions
(±)-Thalidomide selectively inhibits biosynthesis of tumor necrosis factor α (TNF-α). It also functions as an inhibitor of angiogenesis, an immunosuppressive agent, a sedative and a teratogen. Furthermore, thalidomide is known to exhibit antitumor functions in refractory multiple myeloma.
Mechanism of action
Its absorption from the gastrointestinal tract is slow, with peak plasma levels being reached after 3 to 6 hours. It appears to undergo nonenzymatic hydrolysis in the plasma to a large number of metabolites.The elimination half-life is approximately 9 hours.
Pharmacokinetics
Thalidomide, originally developed as a sedative, is an immunomodulatory and anti-inflammatory agent with a spectrum of activity that is not fully characterized. However, thalidomide is believed to exert its effect through inhibiting and modulating the level of various inflammatory mediators, particularly tumor necrosis factor-alpha (TNF-a) and IL-6. Additionally, thalidomide is also shown to inhibit basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), suggesting a potential anti-angiogenic application of thalidomide in cancer patients.
Thalidomide is racemic — it contains both left and right handed isomers in equal amounts: the (+)R enantiomer is effective against morning sickness, and the (?)S enantiomer is teratogenic. The enantiomers are interconverted to each other in vivo; hence, administering only one enantiomer will not prevent the teratogenic effect in humans .
Clinical Use
Thalidomide is approved for use in the United States for the treatment of cutaneous manifestations of erythema nodosum leprosum, a potentially lifethreatening systemic vasculitis that occurs in some patients with leprosy.Although not approved for other indications, thalidomide has also been shown to be very effective in the management of Beh?et’s disease, HIVrelated mucosal ulceration (aphthosis), and select cases of lupus erythematosus.
Side Effects
Thalidomide is a highly teratogenic drug, characteristically
causing phocomelia (aplasia of the midportions of
the limbs). Even a single dose may cause fetal malformation.
Thalidomide should be prescribed to women of
childbearing potential only when no acceptable alternative
exists. Because it is not known whether thalidomide
is present in the ejaculate of males receiving the drug,
male patients must use a latex condom when engaging in
sexual activity with women of childbearing potential.
Other side effects of thalidomide may include sedation
(in fact, thalidomide was originally marketed in
Europe as a sleeping aid), constipation, and peripheral
neuropathy, which may be permanent.
Safety Profile
Poison by ingestion. Moderately toxic by skin contact and intraperitoneal routes. Human teratogenic effects by ingestion: developmental abnormalities of the musculoskeletal and cardiovascular systems. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic and teratogenic data. Human mutation data reported. It was commonly used as a prescription drug in Europe in the late 1950s and early 1960s. Its use was dscontinued because it was lscovered to cause serious congenital abnormalities in the fetus, notably amelia and phocomelia (absence or deformity of the limbs, including hands and feet) when taken by a woman during early pregnancy. When heated to decomposition it emits toxic fumes of NOx. Used as a sedative and hypnotic.
Drug interactions
Potentially hazardous interactions with other drugs
Thalidomide enhances the effects of barbiturates,
alcohol, chlorpromazine and reserpine.
Use with caution with other drugs that can cause
peripheral neuropathy.
Metabolism
Thalidomide appears to undergo primarily non-enzymatic hydrolysis in plasma to multiple metabolites, as the four amide bonds in thalidomide allow for rapid hydrolysis under physiological pH.
Evidences for enzymatic metabolism of thalidomide is mixed, as in vitro studies using rat liver microsome have detected 5-hydroxythalidomide (5-OH), a monohydroxylated metabolite of thalidomide catalyzed by the CYP2C19 enzyme, and the addition of omeprazole, a CYP2C19 inhibitor, inhibits the metabolism of thalidomide. 5-hydroxythalidomide (5-OH) has also been detected in the plasma of 32% of androgen-independent prostate cancer patients undergoing oral thalidomide treatment. However, significant interspecies difference in thalidomide metabolism has been noted, potentially signifying that animals like rats and rabbits rely on enzymatic metabolism of thalidomide more than human.
Metabolism
Thalidomide is metabolised almost exclusively by nonenzymatic hydrolysis. In plasma, unchanged thalidomide
represents 80
% of the circulatory components.
Unchanged thalidomide was a minor component
(<3
% of the dose) in urine. In addition to thalidomide,
hydrolytic products N-(o-carboxybenzoyl) glutarimide
and phthaloyl isoglutamine formed via non-enzymatic
processes are also present in plasma and in urine.
Storage
Store at RT
Toxicity evaluation
The mechanism of teratogenecity of the thalidomide is not clearly understood; however, recent studies have shed some light on the potential mechanism. CRBN has been identified as a thalidomide-binding protein. The teratogenic effects start with binding of thalidomide to CRBN and inhibiting the associated ubiquitin ligase activity.Many E3 ubiquitin ligases are important for various physiological processes such as cell cycle regulation, carcinogenesis, immune response, and development. It has been shown that CRBN forms an E3 ubiquitin ligase complex with damaged DNA binding protein 1 (DDB1) and Cullin-4A (Cul4A), which are important factors for expression of the fibroblast growth factor Fgf8 in zebra fish and chicks as well as the limb outgrowth. In thalidomide-treated zebra fish embryos, formation of proximal endoskeletal disc of the pectoral fin was severely inhibited and the otic vesicle size was significantly reduced. Pectoral fins and otic vesicles in fish share common molecular pathways with tetrapod limbs and ears development. Down-regulation of the CRBN complex causes similar developmental defects in zebra fish. Thalidomide does not cause limb malformations in rodents but does in rabbits, monkeys. In pregnant rats, thalidomide can cause other types of developmental defects such as vertebral column, rib, and eye malformation. There is very strong conservation of the amino acid sequence of CRBN between rat, rabbit, monkey, mouse and human and has been shown that they can bind to thalidomide. The role and importance of CRBN in different species have not been identified; therefore, the degree of teratogenecity could depend in part on the nature of the protein substrates that are modified by CRBN activity during embryonic development. There are some species differences in teratogenecity between thalidomide and its close analogs.
References
1) Ito et al. (2010), Identification of a primary target of thalidomide teratogenicity; Science, 327 1345 2) Weglicki et al. (1993), Inhibition of tumor necrosis factor-alpha by thalidomide in magnesium deficiency; Mol. Cell. Biochem., 129 195 3) D’Amato et al. (1994), Thalidomide is an inhibitor of angiogenesis ; Proc. Natl. Acad. Sci. USA, 91 4082
Properties of Thalidomide
| Melting point: | 269-271°C |
| Boiling point: | 401.48°C (rough estimate) |
| Density | 1.2944 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.6 mg/mL |
| pka | 10.70±0.40(Predicted) |
| form | White solid |
| color | white |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| λmax | 300nm(lit.) |
| Merck | 14,9255 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-35-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Phthalimide, n-(2,6-dioxo-3-piperidyl)-(50-35-1) |
| EPA Substance Registry System | Thalidomide (50-35-1) |
Safety information for Thalidomide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H312:Acute toxicity,dermal |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Thalidomide
| InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
Thalidomide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 THALIDOMIDE 99%View Details
THALIDOMIDE 99%View Details -
 50-35-1 Thalidomide 98%View Details
50-35-1 Thalidomide 98%View Details
50-35-1 -
 Thalidomide 98% CAS 50-35-1View Details
Thalidomide 98% CAS 50-35-1View Details
50-35-1 -
 (±)-Thalidomide CAS 50-35-1View Details
(±)-Thalidomide CAS 50-35-1View Details
50-35-1 -
 Thalidomide CAS 50-35-1View Details
Thalidomide CAS 50-35-1View Details
50-35-1 -
 Thalidomide CAS 50-35-1View Details
Thalidomide CAS 50-35-1View Details
50-35-1 -
 Thalix 50 Capsule ThalidomideView Details
Thalix 50 Capsule ThalidomideView Details
50-35-1 -
 Thalidomide (Thalix 50mg Capsule)View Details
Thalidomide (Thalix 50mg Capsule)View Details
50-35-1
