Pergolide mesylate salt
Synonym(s):8β-[(Methylthio)methyl]-6-propylergoline methanesulfonate salt;Pergolide mesylate salt;Pergolide methanesulfonate salt
- CAS NO.:66104-23-2
- Empirical Formula: C20H30N2O3S2
- Molecular Weight: 410.59
- MDL number: MFCD00153859
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
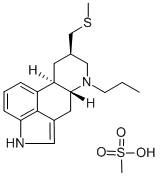
What is Pergolide mesylate salt?
Description
Pergolide mesylate is a potent, long-acting dopamine agonist useful in the treatment of Parkinson’s disease and hyperprolactinemia, Compared with lergotrile, pergolide mesylate has shown less toxicity and lower propensity for inducing psychosis.
Description
Pergolide is a potent dopamine D1 and D2 receptor agonist (Kis = 111 and 0.495 nM, respectively, for rat striatal receptors). It depresses dopaminergic firing in paralyzed rats (ED50 = <20 μg/kg), an effect that can be reversed by the dopamine D2-selective antagonist spiperone or the dopamine D1-selective antagonist SCH 23390 . Pergolide (0.025 and 0.05 mg/kg) increases the volume threshold for inducing bladder contraction in a cynomolgus monkey model of Parkinson''s disease induced by MPTP. It also reduces carrageenan-induced paw edema in adrenalectomized rats (ED50 = 0.4 mg/kg).
Chemical properties
White to Off-White Solid
Originator
Lilly (USA)
The Uses of Pergolide mesylate salt
A dopaminergic agonist that also decrease plasma prolactin concentrations. An antiparkinsonian agent
The Uses of Pergolide mesylate salt
glucocorticoid, antiinflammatory
The Uses of Pergolide mesylate salt
dopamine receptor agonist, anti-Parkinson's agent
What are the applications of Application
Pergolide Mesylate is A dopaminergic agonist
Definition
ChEBI: A methanesulfonate salt obtained from pergolide by mixing eqimolar amount of pergolide and methanesulfonic acid. A dopamine D2 receptor agonist which also has D1 and D2 agonist properties, it i used in the management of Parkinson's disease, although it was withdrawn from the U.S. and Canadian markets in 2007 due to an increased risk of cardiac valve dysfunction.
Manufacturing Process
Dimethyl disulfide (73.6 ml, 0.79 mol) and tri-n-butylphosphine (79.6 ml, 0.32 mol) were added to a solution of 9,10-dihydrolysergol in (8.1 g, 0.032 mol) in the 150 ml of anhydrous DMF and were stirred at room temperature for 6 hours under a nitrogen atmosphere. Dimethyl disulfide of the reaction mixture was removed under vacuo. A solution of the residue in ethyl acetate was extracted with 3.7% HCl (aq.). The aqueous layer was basified with ammonium hydroxide to a pH of 10 and then extracted with ethyl acetate. Removal of ethyl acetate followed by a silica gel column purification eluting with 10% MeOH/CH2Cl2 gave5.5.g of D-6-methyl-8β(methylthiomethyl)ergoline (60%).
A solution of D-6-methyl-8β-(methylthiomethyl)ergoline (0.4 g, 0.0014 mol) and NaI (0.63 g, 0.0042 mol) in 10 ml of propionic anhydride was refluxed for 40 hours. The reaction mixture was guenched with a 10% Na2CO3 solution and extracted by ethyl acetate. The combined organic layers were washed with a saturated brine solution, dried with magnesium sulfate and concentrated to produce oil. The oil was purified by silica gel column, eluting with 10% MeOH/CH2Cl2 to give 0.33 g of D-1,6-dipropionyl-8β(methylthiomethyl)ergoline.
LiAlH4 (0.6 g, 0.0156 mol) was slowly added to a solution of D-1,6dipropionyl-8β-(methylthiomethyl)ergoline in the 20 ml anhydrous THF at 0°C under nitrogene atmosphere. The mixture was stirred at 0°C for 30 min and then at room temperature for 4 hours. The reaction was cooled to 0°C and 0.6 ml of water was slowly added. The mixture was stirred at 0°C for 10 min and 1.8 ml of 15% NaOH (aq.) and 2.5 ml of water were added respectively. The mixture was stirred for 30 min at room temperature and then filtered. Excess of the solvent was removed under reduced pressure to give 150 mg of 8β-((methylthio)methyl)-6-propyl-ergoline or pergolide (yield: 68%). Ergoline,8-((methylthio)methyl)-6-propyl-, monomethanesulfonate, (8β)- may be prepared by mixing of components in solution.
brand name
Permax (Valeant).
Therapeutic Function
Dopamine agonist
Biochem/physiol Actions
Dopaminergic agonist; suppresses pituitary secretion of prolactin; anti-Parkinsonian agent.
Properties of Pergolide mesylate salt
| Melting point: | 252-254°C |
| alpha | aD20 between -18.0° and -23.0° (c = 10 mg/ml in DMF) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | ethanol: soluble2mg/mL |
| form | solid |
| pka | pKa (66% DMF) 7.8(at 25℃) |
| color | white |
| optical activity | [α]20/D 35°, c = 0.2 in pyridine(lit.) |
| CAS DataBase Reference | 66104-23-2(CAS DataBase Reference) |
Safety information for Pergolide mesylate salt
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
Computed Descriptors for Pergolide mesylate salt
| InChIKey | UWCVGPLTGZWHGS-PLBOZXOQNA-N |
| SMILES | C12=C3C=CC=C1[C@@]1([H])C[C@H](CN(CCC)[C@]1([H])CC2=CN3)CSC.S(=O)(=O)(O)C |&1:6,9,15,r| |
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 Pergolide mesylate CAS 66104-23-2View Details
Pergolide mesylate CAS 66104-23-2View Details
66104-23-2 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4