Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
- CAS NO.:16090-14-5
- Empirical Formula: C7F14O4S
- Molecular Weight: 446.12
- MDL number: MFCD00798138
- EINECS: 240-249-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
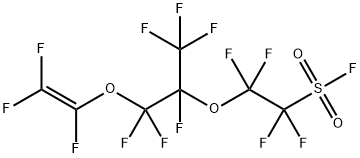
What is Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride?
The Uses of Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes.
The Uses of Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl Fluoride is used as a reagent in the synthesis of novel perfluorinated vinyl ether containing sulfonimide functionality which can be copolymered with tetrafluoroethylene to yield a novel perfluorinated copolymer.
Synthesis

(1) Intermediate synthesis In a 1L reactor, add 100ml of dimethylacetamide, 8g of potassium fluoride, and 200g (1.11mol) of tetrafluoroethane-|?-sultone,Subsequently, 500g (3mol) of hexafluoropropylene oxide was introduced at a flow rate of 0.3kg/h for addition reaction, and the reaction temperature was controlled at 20??C.The reaction pressure is controlled at 0.4MPa. After the introduction of hexafluoropropylene oxide is completed, the reaction is continued for 1 hour. After the reaction is completed,The reaction liquid was rectified to obtain 528g (1.03mol) of intermediate, with a yield of 92% and a purity of 99.1%;(2) PSVE synthesis In a moving bed reactor (volume 2L, material 316L, produced by Tianjin Qixi Technology Co., Ltd.),A mixture of 500g (0.97mol) of the intermediate obtained in step (1) and 600g of potassium carbonate (4.34mol) was passed through the salt formation zone and decarboxylation zone of the moving bed reactor in turn (the salt formation temperature was set to 150??C,The decarboxylation temperature is set to 360??C) to carry out the salt formation reaction and the decarboxylation reaction, and the contact time of the salt formation reaction is 10 min by controlling the material feed rate.The contact time of the decarboxylation reaction is 25min, and the decarboxylation reaction product is collected by condensation to obtain a crude product.The crude product is rectified to obtain the product perfluoro-3,6-dioxa-4-methyl-7-octenesulfonyl fluoride with a purity of 98.5% and a yield of 85%.
Properties of Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
| Boiling point: | 135°C |
| Density | 1,7 g/cm3 |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Colourless |
| EPA Substance Registry System | Ethanesulfonyl fluoride, 2-[1-[difluoro[(trifluoroethenyl)oxy]methyl]-1,2,2,2-tetrafluoroethoxy]-1,1,2,2-tetrafluoro- (16090-14-5) |
Safety information for Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran


You may like
-
 Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride 95% CAS 16090-14-5View Details
Perfluoro(4-methyl-3,6-dioxaoct-7-ene)sulfonyl fluoride 95% CAS 16090-14-5View Details
16090-14-5 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3