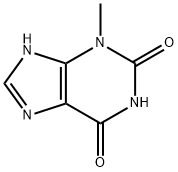
Theobromine
Synonym(s):2,6-Dihydroxy-3,7-dimethylpurine;3,7-Dihydro-3,7-dimethyl-1H-purine-2,6-dione;3,7-Dimethylxanthine;Theobromine
- CAS NO.:83-67-0
- Empirical Formula: C7H8N4O2
- Molecular Weight: 180.16
- MDL number: MFCD00022830
- EINECS: 201-494-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
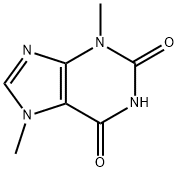
What is Theobromine?
Description
Theobromine is a methylxanthine alkaloid and derivative of caffeine that has been found in cocoa beans and has diverse biological activities. It is an adenosine A1 receptor antagonist (IC50s = 200-280 μM in radioligand binding assays using rat brain membranes). Theobromine (150 μg/ml) increases AMPK phosphorylation and inhibits adipocyte differentiation, ERK and JNK phosphorylation, and IL-6 and TNF-α production in 3T3-L1 preadipocytes cultured in differentiation medium. It inhibits decreases in renal cortex SIRT1 activity and increases in NADPH oxidase-dependent reactive oxygen species (ROS) production, as well as reduces kidney hypertrophy and albuminuria in a spontaneously hypertensive rat model of streptozotocin-induced diabetes when administered at a dose of 5 mg/kg per day. Theobromine is toxic to dogs with an LD50 value of 250 to 500 mg/kg.
Description
Theobromine is the primary alkaloid in chocolate—it gives unsweetened chocolate its bitter taste. It gets its name from the cacao tree genus?Theobroma, but it’s also found in tea leaves and kola nuts. A. Woskresensky discovered theobromine in cacao beans in 1841, and E. Fischer synthesized it in 1882. If you replace the nitrogen-bearing hydrogen group with a methyl group, you have caffeine.
Description
A purine alkaloid which is present in a number of plants, particularly Theobroma
coco and kola nuts. The alkaloid sublimes at 290°C and may be purified by this
method when it forms colourless, rhombic microcrystals. It is soluble in 1600
parts of H20 at 17°C, insoluble in cold Et20 or ligroin. It is a weak base and the
salts are decomposed by H20. The perchlorate forms colourless crystals of the
monohydrate which decompose at 271-3°C; the mercurinitrate is also crystalline
and has a melting point above 300°C. The mercury salt is obtained as colourless
crystals which darken at 29S-300°C and melt at 310°C. One imino group is
present yielding alkyl derivative, e.g. the N-methyl compound identical with
Caffeine (q.v.); the N~ethyl derivative, m.p. 164-SoC, forming an aurichloride,
m.p. 226°C; N-propyl, m.p. 136°C giving an aurichloride, m.p. 9SoC; N-butyl,
m.p. 119°C and the N-iso butyl, m.p. 129-130oC also giving an aurichloride,m.p. 97°C. The alkaloid also furnishes a methochloride as colourless rods, m.p.
320-340°C (dec.) which is very soluble in H20 and the methoaurichloride, m.p.
265°C (dec.).
Like Caffeine (q.v.) and Theophylline (q.v.), the alkaloid and its salts are
mild stimulants and also possess a diuretic action.
Chemical properties
white to light yellow crystal powder
Chemical properties
A methyl xanthine similar to caffeine. Theobromine has a bitter taste.
Occurrence
Reported found in cocoa bean, cocoa powder, cola nut and tea.
The Uses of Theobromine
A metabolite of Caffeine
The Uses of Theobromine
diuretic, bronchodilator, cardiotonic
The Uses of Theobromine
A metabolite of Caffeine.
What are the applications of Application
Theobromine is a weak phosphodiesterase inhibitor and adenosine receptor blocker
Definition
ChEBI: A dimethylxanthine having the two methyl groups located at positions 3 and 7. It is a purine alkaloid derived from cocoa tree.
General Description
Odorless white crystalline powder. Bitter taste. pH (saturated solution in water): 5.5-7.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Theobromine may be sensitive to prolonged exposure to light. Theobromine has weakly acidic properties, combining with bases to forms salts. Theobromine also has even weaker basic properties, combining with acids to form salts which are decomposed in aqueous solution. .
Hazard
Toxic by ingestion. Questionable carcinogen.
Fire Hazard
Flash point data for Theobromine are not available; however, Theobromine is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by ingestion: central nervous system and gastrointestinal changes. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx. Used as a diuretic, smooth muscle relaxant, cardiac stimulant, and vasoddator.
Purification Methods
It crystallises from H2O. Its solubility in H2O is 0.06% at 15o and 1.25% at 100o, and it is poorly soluble in organic solvents. It forms salts with heavy metals and is a diuretic, vasodilator and a cardiac stimulant. [Lister Purines Part II, Fused Pyrimidines Brown Ed, Wiley-Interscience pp254-225 1971, ISBN 0-471-38205-1, Beilstein 26 H 457, 26 I 135, 26 II 264, 26 III/IV 2336.]
References
Biltz, Max., Annalen, 423,320 (1921)
Dubosc., Chem. Zentr., IV, 956 (1932)
Gepner, Kreps., Chem. Abstr., 41,96 (1947)
Bohinc, Korber-Smid, J agodic., Farm. Vestn., (Ljubljana), 23, 143 (1972)
Properties of Theobromine
| Melting point: | 345-350 °C |
| Boiling point: | 312.97°C (rough estimate) |
| Density | 1.50 |
| refractive index | 1.6700 (estimate) |
| FEMA | 3591 | THEOBROMINE |
| Flash point: | 290-295°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: slightly soluble |
| form | solid |
| pka | 7.89(at 18℃) |
| color | white |
| Odor | odorless |
| Water Solubility | slightly soluble, <0.1 g/100 mL at 18 ºC |
| Merck | 14,9282 |
| Sublimation | 290-295 ºC |
| BRN | 16464 |
| CAS DataBase Reference | 83-67-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Purine-2,6-dione, 3,7-dihydro-3,7-dimethyl-(83-67-0) |
| IARC | 3 (Vol. 51) 1991 |
| EPA Substance Registry System | Theobromine (83-67-0) |
Safety information for Theobromine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Theobromine
| InChIKey | YAPQBXQYLJRXSA-UHFFFAOYSA-N |
Theobromine manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
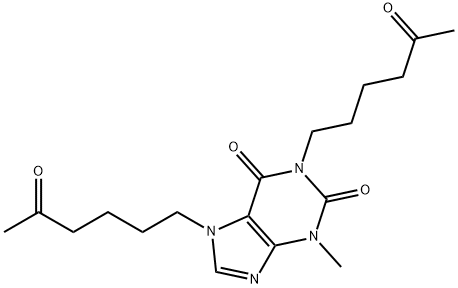
![3,7-Dihydro-3,7-diMethyl-6-[(5-oxohexyl)oxy]-2H-purin-2-one](https://img.chemicalbook.in/CAS/GIF/93079-86-8.gif)
![1,1'-[(5E)-5-Methyl-7-oxo-5-undecene-1,11-diyl] Bis](https://img.chemicalbook.in/CAS/GIF/874747-30-5.gif)
![1,1'-Methylene Bis[TheobroMine]](https://img.chemicalbook.in/CAS/GIF/77196-87-3.gif)

You may like
-
 Theobromine 98%View Details
Theobromine 98%View Details
83-67-0 -
 Theobromine 98.00% CAS 83-67-0View Details
Theobromine 98.00% CAS 83-67-0View Details
83-67-0 -
 Theobromine 98% CAS 83-67-0View Details
Theobromine 98% CAS 83-67-0View Details
83-67-0 -
 Theobromine CAS 83-67-0View Details
Theobromine CAS 83-67-0View Details
83-67-0 -
 Theobromine CAS 83-67-0View Details
Theobromine CAS 83-67-0View Details
83-67-0 -
 TheobromineView Details
TheobromineView Details
83-67-0 -
 Theobromine, Grade Standard: Industrial, Packaging Type: BagView Details
Theobromine, Grade Standard: Industrial, Packaging Type: BagView Details
7790-21-8 -
 TheobromineView Details
TheobromineView Details
83-67-0
