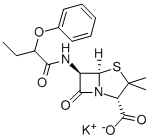
Penicillin V potassium salt
Synonym(s):Penicillin V potassium salt;Phenoxymethylpenicillin potassium salt
- CAS NO.:132-98-9
- Empirical Formula: C16H17KN2O5S
- Molecular Weight: 388.48
- MDL number: MFCD00051771
- EINECS: 205-086-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
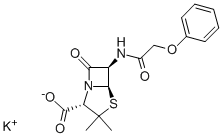
What is Penicillin V potassium salt?
Chemical properties
Crystalline Solid
Originator
Oracilline ,Theraplix ,France ,1954
The Uses of Penicillin V potassium salt
Penicillin antibacterial.
The Uses of Penicillin V potassium salt
Penicillin V Potassium bactericidal against penicillin-susceptible microorganisms during the stage of active multiplication. It inhibits biosynthesis of cell-wall mucopeptide. It is used to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
What are the applications of Application
Penicillin V Potassium is a bactericidal agent
Definition
ChEBI: Phenoxymethylpenicillin potassium is an organic potassium salt. It contains a phenoxymethylpenicillin(1-).
What are the applications of Application
Phenoxymethylpenicillin potassium was first obtained at Lilly Research Laboratories in 1948, following the addition of phenoxyacetic acid to a culture of Penicillium chrysogenum. Its industrial-scale production was established in 1953. Its usefulness as an orally active penicillin was suggested by Spitzy et al. in 1955. It is less hygroscopic and much more stable against gastric acid than benzylpenicillin, and it has been used orally for therapy of infections caused by Streptococcus, Staphylococcus, and other gram-positive bacteria as well as Neisseria and Leptospira.
Manufacturing Process
The following description is taken from US Patent 2,941,995. A solution of phenoxyacetyl chloride (360 mg) in dry acetone (5 ml) was added dropwise during 10 minutes to a stirred solution of 6-aminopenicillanic acid (450 mg, approximately 75% pure) in 3% aqueous bicarbonate (18 ml), and acetone (12 ml). When addition was complete the mixture was stirred at room temperature for 30 minutes and then extracted with ether (30 ml in 3 portions), only the aqueous phase being retained. This aqueous solution was covered with butanol (5 ml) and adjusted to pH 2 by the addition of N hydrochloric acid. After separating the layers, the aqueous phase was extracted with two 2.5 ml portions of butanol, adjusting to pH 2 each time. The combined butanol solutions (which at this stage contained the free penicillanic acid) were washed with water (3 x 2 ml) and then shaken with water (10 ml) to which sufficient 3% sodium bicarbonate solution was added to bring the aqueous phase to pH 7. The butanol solution was further extracted with two 5 ml portions of water to each of which was added enough bicarbonate solution to produce an aqueous phase of pH 7. The combined aqueous solutions were washed with ether (20 ml) and then evaporated at low temperature and pressure to leave the crude sodium salt of phenoxymethyl penicillin which, after drying in a vacuum desiccator, was obtained as a slightly hygroscopic powder (591 mg).
brand name
V-Cillin(Lilly).
Therapeutic Function
Antibacterial
General Description
Odorless white crystalline powder. Slightly bitter taste. pH (0.5% aqueous solution) 5 to 7.5.
Air & Water Reactions
Water soluble.
Reactivity Profile
Penicillin V potassium salt is the potassium salt of penicillin v. Penicillin V potassium salt is incompatible with acids, oxidizing agents (especially in the presence of trace metals), heavy metal ions such as copper, lead, zinc and mercury; glycerol, sympathomimetic amines, thiomersal, wood alcohols, cetostearyl alcohol, hard paraffins, macrogols, cocoa butter and many ionic an nonionic surface-active agents. Penicillin V potassium salt is also incompatible with alkalis, compounds leached from vulcanized rubber, hydrochlorides of tetracyclines and organic peroxides. Other incompatibilities include reducing agents, alcohols, other hydroxy compounds, self-emulsifying stearyl alcohol, emulsifying wax, lanolin, crude cholinesterated bases, glycol, sugars, amines, aminacrine hydrochloride, ephedrine, procaine, rubber tubing, thiamine hydrochloride, zinc oxide, oxidized cellulose, iodine, iodides, thiols, chlorocresol and resorcinol. Penicillin V potassium salt may also be incompatible with naphthalene oils and vitamin B.
Fire Hazard
Flash point data for Penicillin V potassium salt are not available; however, Penicillin V potassium salt is probably combustible.
Safety Profile
Moderately toxic by ingestion, intramuscular, intraperitoneal, and intravenous routes. Human mutation data reported. Questionable carcinogen with no experimental evidence. When heated to decomposition it emits very toxic fumes of NOx, K2O, and SOx. Used
Properties of Penicillin V potassium salt
| Melting point: | 197-202°C |
| alpha | D25 +223° (c = 0.2) |
| Density | 1.40 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Freely soluble in water, practically insoluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | Sol in water |
| Water Solubility | Soluble in water, dimethyl sulfoxide, and methanol. |
| BRN | 3899451 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 132-98-9(CAS DataBase Reference) |
| EPA Substance Registry System | Penicillin V Potassium (132-98-9) |
Safety information for Penicillin V potassium salt
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Penicillin V potassium salt
| InChIKey | HCTVWSOKIJULET-LQDWTQKMSA-M |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Phenoxymethylpenicillin potassium CAS 132-98-9View Details
Phenoxymethylpenicillin potassium CAS 132-98-9View Details
132-98-9 -
 Penicillin V Potassium Salt CAS 132-98-9View Details
Penicillin V Potassium Salt CAS 132-98-9View Details
132-98-9 -
 Penicillin V Potassium Salt CAS 132-98-9View Details
Penicillin V Potassium Salt CAS 132-98-9View Details
132-98-9 -
 Penicillin V Potassium Salt Cas 132 98 9View Details
Penicillin V Potassium Salt Cas 132 98 9View Details
132-98-9 -
 Penicillin V Potassium Salt CAS 132-98-9View Details
Penicillin V Potassium Salt CAS 132-98-9View Details
132-98-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
