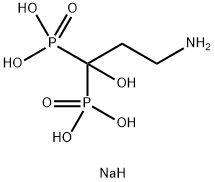
Pamidronate disodium salt
Synonym(s):3-Amino-1-hydroxy-1-phosphonopropanephosphonic acid disodium salt hydrate
- CAS NO.:57248-88-1
- Empirical Formula: C3H12NNaO7P2
- Molecular Weight: 259.07
- MDL number: MFCD01938386
- EINECS: 260-647-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Pamidronate disodium salt?
Description
Disodium pamidronate is a calcium metabolism regulator useful in the treatment of hypercalcemia associated with malignancy. It presumably reduces the bone resorption of calcium by inhibiting the formation and attachment of osteoclasts. Other potential uses include osteoporosis and calcitonin-resistant Paget’s disease.
Chemical properties
White Crystals
Originator
Henkel (United Kingdom)
The Uses of Pamidronate disodium salt
A bisphosphonate antiresorptive agent; bone resorption inhibitor.
The Uses of Pamidronate disodium salt
Pamidronate disodium is a nitrogen containing bisphosphonate, used to prevent osteoporosis. It is used to prevent bone loss, and treat osteoporosis.
The Uses of Pamidronate disodium salt
A farnesyl diphosphate synthase inhibitor
What are the applications of Application
Pamidronate disodium salt is a farnesyl diphosphate synthase inhibitor
Manufacturing Process
For a batch size of 5 L, 587.5 g (3.2 moles) of mannitol is dissolved in 3.5 L of water. Pamidronic acid (31.6 g, 0.133 moles) is mixed with a 1.0 L aliquot of the mannitol solution to form a slurry. The slurry is then transferred into the remainder of the mannitol solution, and stirred for at least 15 min. Aqueous 1 N sodium hydroxide (270 ml) is then added and the mixture is stirred until a clear, colorless solution results. The pH is then adjusted to 6.50.1 using either 1 M aqueous phosphoric acid or 1 N aqueous sodium hydroxide, as needed. The solution is then filtered through a 0.22 micron filter, and filled at 20°C into vials at 4.0 ml (4.172 g)/vial, under sterile conditions. The aqueous solution is frozen at -37°C and lyophilized (20 mbar, 20°-40°C) to yield 1,250 vials, each containing 30 mg of amorphous disodium pamidronate. The vials are sealed under positive nitrogen pressure. The disodium pamidronate is amorphous (noncrystalline) by X-ray diffraction and contains 0.7 wt-% water.
brand name
Aredia (Novartis).
Therapeutic Function
Bone resorption suppressant
Biochem/physiol Actions
Pamidronate disodium?has the ability to block Wnt and β-catenin signaling, which modulates the osteogenic differentiation in bone marrow mesenchymal stem cells (BMMSCs). It can also reduce bilirubin-impaired apoptosis and helps to develop dentinogenic dysfunction of stem cells from human deciduous teeth.
Veterinary Drugs and Treatments
Pamidronate may be useful in treating hypercalcemia associated with vitamin D-related toxicoses or hypercalcemia of malignancy. There is ongoing research on the use of this drug to determine if it has clinical usefulness in directly treating “micro-metastases” in osteosarcomas.
Properties of Pamidronate disodium salt
| Melting point: | 300 °C |
| storage temp. | 2-8°C |
| solubility | H2O: 28 mg/mL |
| form | solid |
| color | white |
| Merck | 14,7003 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 57248-88-1(CAS DataBase Reference) |
Safety information for Pamidronate disodium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Pamidronate disodium salt
Pamidronate disodium salt manufacturer
Rivashaa Agrotech Biopharma Pvt. Ltd.
Ralington Pharma
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 57248-88-1 98%View Details
57248-88-1 98%View Details
57248-88-1 -
 57248-88-1 Pamidronate disodium 98%View Details
57248-88-1 Pamidronate disodium 98%View Details
57248-88-1 -
 Pamidronate disodium 97% CAS 57248-88-1View Details
Pamidronate disodium 97% CAS 57248-88-1View Details
57248-88-1 -
 Sodium (3-amino-1-hydroxy-1-phosphonopropyl)phosphonate hydrate 95% CAS 57248-88-1View Details
Sodium (3-amino-1-hydroxy-1-phosphonopropyl)phosphonate hydrate 95% CAS 57248-88-1View Details
57248-88-1 -
 Disodium pamidronate hydrate 95% CAS 57248-88-1View Details
Disodium pamidronate hydrate 95% CAS 57248-88-1View Details
57248-88-1 -
 Disodium Pamidronate CAS 57248-88-1View Details
Disodium Pamidronate CAS 57248-88-1View Details
57248-88-1 -
 Pamidronate disodium salt hydrate CAS 57248-88-1View Details
Pamidronate disodium salt hydrate CAS 57248-88-1View Details
57248-88-1 -
 Disodium pamidronate CAS 57248-88-1View Details
Disodium pamidronate CAS 57248-88-1View Details
57248-88-1