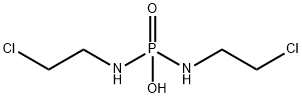
Palifosfamide
- CAS NO.:31645-39-3
- Empirical Formula: C4H11Cl2N2O2P
- Molecular Weight: 221.02
- MDL number: MFCD01705455
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Palifosfamide?
Description
Palifosfamide is an active metabolite of the DNA alkylating agent ifosfamide . Palifosfamide is formed through hydroxylation of ifosfamide by various hepatic cytochrome P450 (CYP) isoforms. It is cytotoxic to L1210 and CCRF-CEM cancer cells when used at a concentration of 100 μM. Palifosfamide (100 mg/kg per day) reduces tumor growth in murine Lewis lung carcinoma and L1210 leukemia models, as well as a rat Yoshida ascitic sarcoma model.
Definition
ChEBI: Palifosfamide, also known as Isophosphamide mustard, is a type of phosphorodiamide. It is a proprietary stabilized metabolite of ifosfamide. Ifosfamide has been shown to be effective in high doses in treating testicular cancer, sarcoma and lymphoma.
in vitro
Palifosfamide lysine (ZIO-201) is a stable form of palifosfamide. It has broad activity in sarcoma lines in vitro. The IC50 ranges from 2.25 ro 6.75 μM for most cell lines except OS222 (IC50=31.5 μM).
in vivo
Tumor growth inhibition is seen in both OS31 and OS33 xenografts and the RMS xenograft resulting in a significant difference in event-free survival between the control and the treated groups. Differential gene expression of ALDH3A1 but not ALDH1A1 is noted in the OS31 xenograft. Stabilized palifosfamide administered to mice suppresses MX-1 tumor growth by greater than 80% with 17% complete antitumor responses. Oral bioavailability in rats is 48-73% of parenteral administration, and antitumor activity in mice is equivalent by both routes. Treatment with palifosfamide-tris combined with docetaxelor doxorubicin at optimal regimens results in complete tumor regression in 62-75% of mice.
Mode of action
Palifosfamide works by irreversibly alkylating and cross-linking DNA through GC base pairs, leading to 7-atom inter-strand cross-links that cannot be repaired. Its cytotoxic effect primarily stems from its ability to cross-link strands of DNA and RNA, inhibit protein synthesis, and block DNA replication, ultimately causing cell death. Unlike ifosfamide, this agent is not metabolized to acrolein or chloroacetaldehyde, metabolites associated with bladder and CNS toxicities.
References
https://go.drugbank.com/drugs/DB05668
https://sarcomahelp.org/articles/palifosfamide.html
https://pubchem.ncbi.nlm.nih.gov/compound/Palifosfamide
Properties of Palifosfamide
| Melting point: | 106-107 ºC |
| Boiling point: | 341.5±52.0 °C(Predicted) |
| Density | 1.411 |
| storage temp. | Store at -20°C, protect from light, stored under nitrogen |
| solubility | ≥22.1 mg/mL in DMSO; insoluble in EtOH; ≥19.4 mg/mL in H2O with gentle warming and ultrasonic |
| form | Powder |
| pka | 0.79±0.50(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C4H11Cl2N2O2P/c5-1-3-7-11(9,10)8-4-2-6/h1-4H2,(H3,7,8,9,10) |
| CAS DataBase Reference | 31645-39-3 |
Safety information for Palifosfamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Palifosfamide
| InChIKey | BKCJZNIZRWYHBN-UHFFFAOYSA-N |
| SMILES | P(NCCCl)(NCCCl)(O)=O |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8