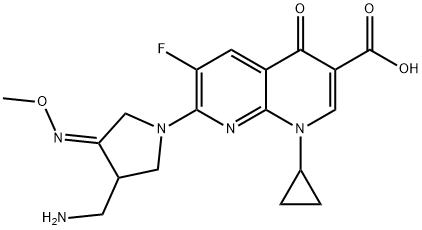
Ozenoxacin
- CAS NO.:245765-41-7
- Empirical Formula: C21H21N3O3
- Molecular Weight: 363.41
- MDL number: MFCD18633255
- EINECS: 105-745-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
What is Ozenoxacin?
Absorption
Four studies were performed in which varying strengths of ozenoxacin cream, up to 2% (twice the concentration of the marketed formulation), were administered to 110 patients. Three of the studies examined systemic absorption in healthy subjects and in subjects having impetigo. The studies were performed with either single or repeated application of up to 1 g ozenoxacin cream to intact or abraded skin (up to 200 cm squared surface area). No systemic absorption was seen in 84 of 86 subjects, and negligible systemic absorption was seen at the level of detection (0.489 ng/mL) in 2 subjects .
Toxicity
Ozenoxacin cream has the potential to cause rosacea and seborrheic dermatitis when administered topically on a patient. In clinical trials, only one adult patient treated with ozenoxacin cream reported such adverse reactions .
Much like other topical antibiotics, the prolonged use of ozenoxacin cream can result in the overgrowth of nonsusceptible bacteria and fungi. If such overgrowths develop into infections during therapy, discontinue use of the ozenoxacin cream and institue appropriate therapy for the infections .
Although clinical studies demonstrate negligible systemic absorption after topical administration of ozenoxacin, there are no formal available data on the use of ozenoxacin in pregnant women to inform any professional drug associated risk for use in pregnancy .
Although breastfeeding is not expected to result in exposure of the child to ozenoxacin owing to the negligible systemic absorption of ozenoxacin in humans following topical administration, no formal data are available regarding the presence of ozenoxacin in human milk and the effects of ozenoxacin on breasfed infants or on milk production .
Clinical experience with ozenoxacin cream has not identified differences in responses between elderly and younger patients .
The safety and effectiveness of ozenoxacin cream in pediatric patients 2 months and older is similar to that of adults, but the safety and effectiveness of ozenoxacin in pediatric patients younger than 2 months of age has not been formally establlished .
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with ozenoxacin .
Ozenoxacin demonstrated no genotoxicity when evaluated in vitro for gene mutation and/or chromosomal effects in the Ames test, mouse lymphoma cell assay, or when evaluated in vivo in a rat micronucleus test with demonstrated systemic exposure .
Oral doses of ozenoxacin did not affect mating and fertility in male and female rats treated up to 500 mg/kg/day (about 8500 and 16,000 times respectively, the maximum human plasma concentration seen with dermal application of ozenoxacin 1% cream) .
Description
Ozenoxacin is a novel, nonfluorinated quinolone antibiotic discovered by Toyama Chemical Co. Ltd. and developed by Maruho Co. Ltd. Ozenoxacin was approved by the PMDA of Japan in September 2015 for the treatment of acne and skin infections. Ozenoxacin shows potent antibacterial activity against anaerobic and aerobic, gram-positive and -negative bacteria, especially those implicated in superficial skin infections such as S. aureus, Staphylococcus epidermidis, and Propionibacterium acnes. The mechanism of action of ozenoxacin involves the drug’s affinity for DNA gyrase and DNA topoisomerase IV and upon binding triggers bacterial apoptosis.
The Uses of Ozenoxacin
Ozenoxacin is a non-fluorinated topical quinolone. It exhibits antimicrobial activity against?propionibacteria and staphylococci. Ozenoxacin can be used to treat acne and superficial skin infections.
Indications
Ozenoxacin cream is indicated for the topical treatment of impetigo caused by Staphylococcus aureus or Streptococcus pyogenes in patients aged 2 months of age and older .
Background
To date, ozenoxacin has been used in trials studying the treatment of impetigo.
As of December 11, 2017 the FDA approved Ferrer Internacional S.A.'s Xepi (ozenoxacin 1%) as a topically applied cream indicated for the treatment of impetigo caused by Staphylococccus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older.
Despite being a common and highly contagious bacerial skin infection that affects millions of children and adults in the United States each year, ozenoxacin cream is a novel, non-fluorinated quinolone that has demonstrated safe and effective therapy in both the adult and pediatric population.
Definition
ChEBI: Ozenoxacin is a member of quinolines.
Pharmacokinetics
Although the exposure response relationship for ozenoxacin after it has been applied topically has not yet been studied, a formal relationship is unlikely because systemic exposure of ozenoxacin following its topical application has been measured to be negligible .
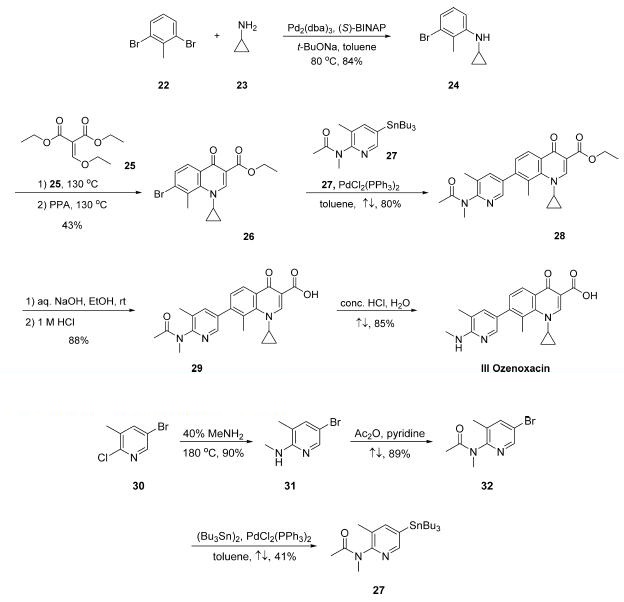
Synthesis
A U.S. patent filed by co-workers at Toyama describes the
only publicly disclosed synthetic approach to this drug.12 The
drug?ˉs assembly hinges upon a key Stille coupling between a
quinolonyl bromide and a stannylpyridine.
Buchwald-Hartwig coupling of commercially available 2,6-
dibromotoluene (22) and cyclopropylamine (23) gave Ncyclopropyl-
3-bromo-2-methylaniline 24 in 84% yield , and this step was followed by reaction with diethyl
ethoxymethylenemalonate (25) and subsequent cyclization
under acidic conditions to secure bromoquinoline 26 in 43%
yield over the two-step sequence. Stille coupling of 27 with
bromoquinoline 26 resulted in pyridyl quinoline adduct 28 in
80% yield. Saponification of ester 28 followed by acidic removal
of the N-acetyl group delivered the active pharmaceutical
ingredient ozenoxacin (III) in 75% yield.
The preparation of key stannane 27, which is not
commercially available and began
with the conversion of commercially available 5-bromo-2-
chloro-3-methylpyridine (30) to aminopyridine derivative 31
upon treatment with aqueous methylamine at elevated
temperature in a sealed vessel. The resulting aminopyridine
was subjected to acetic anhydride in pyridine, resulting in
acetamide 32 in good yield, and this coupling was followed by a
modest-yielding palladium-catalyzed installation of the stannyl
group to deliver subunit 27.
Metabolism
Studies have demonstrated that ozenoxacin is not metabolized in the presence of fresh human skin discs and is minimally metabolized in human hepatocytes .
Properties of Ozenoxacin
| Melting point: | >255°C (dec.) |
| Boiling point: | 573.5±50.0 °C(Predicted) |
| Density | 1.372±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly) |
| form | Solid |
| pka | 6.46±0.50(Predicted) |
| color | Off-White |
Safety information for Ozenoxacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ozenoxacin
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Ozenoxacin 99%View Details
Ozenoxacin 99%View Details -
 Ozenoxacin 99%View Details
Ozenoxacin 99%View Details -
 Ozenoxacin 98%View Details
Ozenoxacin 98%View Details -
 Ozenoxacin 245765-41-7 99%View Details
Ozenoxacin 245765-41-7 99%View Details
245765-41-7 -
 245765-41-7 Ozenoxacin 98%View Details
245765-41-7 Ozenoxacin 98%View Details
245765-41-7 -
 Ozenoxacin CAS 245765-41-7View Details
Ozenoxacin CAS 245765-41-7View Details
245765-41-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6