Oxipurinol
- CAS NO.:2465-59-0
- Empirical Formula: C5H4N4O2
- Molecular Weight: 152.11
- MDL number: MFCD00047205
- EINECS: 219-570-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-14 15:27:00
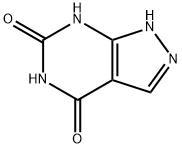
What is Oxipurinol?
Chemical properties
Off White Solid
The Uses of Oxipurinol
A metabolite of Allopurinol.
The Uses of Oxipurinol
4,6-Dihydroxy-1H-pyrazolo[3,4-d]pyrimidine is an inhibitor to study the specificity and kinetics of xanthine oxidase. It is also used as an anti-inflammatory agent via inhibition of xanthine oxidase.
The Uses of Oxipurinol
Oxypurinol, an allopurinol metabolite, is used as an inhibitor to study the specificity and kinetics of of xanthine oxidase(s). Oxypurinol is also used as an anti-inflammatory agent via inhibition of xanthine oxidase.
What are the applications of Application
Oxipurinol is a metabolite of allopurinol used as an inhibitor to study the specificity and kinetics of of xanthine oxidase(s)
Definition
ChEBI: A pyrazolopyrimidine that is 4,5,6,7-tetrahydro-H-pyrazolo[3,4-d]pyrimidine substituted by oxo groups at positions 4 and 6.
Biological Activity
oxipurinol is a xanthine oxidoreductase inhibitor.xanthine oxidoreductase (xo), a complex molybdoflavoenzyme present in milk and many other tissues, has been studied for many years. xo is generally recognized as a critical enzyme in purine catabolism.
Biochem/physiol Actions
An allopurinol metabolite.
in vitro
allopurinol could be rapidly oxidized by xo to its active metabolite oxypurinol (both isosteres of hypoxanthine and xanthine, respectively), which also could inhibit xo. oxypurinol was identified as a noncompetitive inhibitor of xo; the formation of this compound was reported to be responsible for much of the pharmacological activity of allopurinol. moreover, both allopurinol and oxypurinol showed free radical scavenging effects in isolated hearts, and exerted cardioprotective effects despite no detectable xo activities [1].
in vivo
animal study found that in the vasculature of hypercholesterolemic rabbits, oxypurinol treatment led to a decrease in vascular free radical production [1].
References
[1] p. pacher, a. nivorozhkin and c. szabó. therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. pharmacological reviews 58(1), 87-114 (2006).
[2] day ro, graham gg, hicks m, mclachlan aj, stocker sl, williams km. clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. clin pharmacokinet. 2007;46(8):623-44.
Properties of Oxipurinol
| Melting point: | 300 °C |
| Boiling point: | 274.55°C (rough estimate) |
| Density | 1.637 |
| refractive index | 1.8500 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO. |
| pka | pKa 7.7 (Uncertain) |
| form | Powder |
| color | Light yellow |
| BRN | 139956 |
| Stability: | Hygroscoipic |
| CAS DataBase Reference | 2465-59-0(CAS DataBase Reference) |
| EPA Substance Registry System | Oxypurinol (2465-59-0) |
Safety information for Oxipurinol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Oxipurinol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![1-PHENYL-1H-PYRAZOLO[3,4-D]PYRIMIDINE-4,6(5H,7H)-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB8412423.gif)

![1-[3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]-1H-PYRAZOLO[3,4-D]PYRIMIDINE-4,6(5H,7H)-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB4325657.gif)
![1-BENZYL-5-PHENYL-1,7-DIHYDRO-PYRAZOLO[3,4-D]PYRIMIDINE-4,6-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure20/GIF/CB8799605.gif)
![9-[(2-Acetoxyethoxy)methyl]-N2-acetylguanine](https://img.chemicalbook.in/CAS/GIF/75128-73-3.gif)
You may like
-
 2465-59-0 Oxypurinol 99%View Details
2465-59-0 Oxypurinol 99%View Details
2465-59-0 -
 2465-59-0 98%View Details
2465-59-0 98%View Details
2465-59-0 -
![4,6-Dihydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 2465-59-0](https://img.chemicalbook.in//Content/image/CP5.jpg) 4,6-Dihydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 2465-59-0View Details
4,6-Dihydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 2465-59-0View Details
2465-59-0 -
 Oxypurinol 98.00% CAS 2465-59-0View Details
Oxypurinol 98.00% CAS 2465-59-0View Details
2465-59-0 -
 Oxypurinol CAS 2465-59-0View Details
Oxypurinol CAS 2465-59-0View Details
2465-59-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
