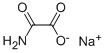
Oxamic acid sodium salt
Synonym(s):Aminooxoacetic acid sodium salt;Oxalic acid monoamide sodium salt;Oxamic acid sodium salt
- CAS NO.:565-73-1
- Empirical Formula: C2H2NNaO3
- Molecular Weight: 111.03
- MDL number: MFCD00044553
- EINECS: 209-290-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Oxamic acid sodium salt?
Description
Oxamic acid sodium salt is a lactate dehydrogenase-A (LDH-A) inhibitor. Oxamic acid sodium salt shows anti-tumor activity, and anti-proliferative activity against cancer cells, and can induce apoptosis. Oxamic acid suppresses the proliferation, migration and invasion of both A2780 and SKOV3 cells.
Chemical properties
white crystalline powder
The Uses of Oxamic acid sodium salt
Oxamic acid sodium salt is a lactate dehydrogenase (LDH) inhibitor. It is a suitable compound to investigate cancer cells that utilize the glycolytic pathway.This compound is suitable for lactate dehydrogenase (LDH) related research. It can be used as an inhibitor of gluconeogenesis useful for anticancer research.
What are the applications of Application
Sodium oxamate is an inhibitor of gluconeogenesis useful for anticancer research
What are the applications of Application
Sodium oxamate, an inhibitor of LDH-C4, prevented the AR induced by lysophosphatidylcholine (LPC) or NADH. Oxamic acid sodium salt is an lactic acid dehydrogenase. Sperm suspension and cytosol fraction activities were inhibited by sodium oxamate. As cancer cells are often dependent on glycolysis for ATP production, sodium oxamate has implications as an anticancer compound.
Biochem/physiol Actions
Sodium oxamate influences the fatty acid metabolism. It might be useful for the treatment of diabetes.
in vitro
Oxamic acid suppresses the proliferation, migration and invasion of both A2780 and SKOV3 cells. Oxamic acid (10 μM; 24-72 h) inhibits cell proliferation in a dose- and time-dependent manner in both NPC cancer cells. Oxamic acid (0-100 mM; 24 h) induces cell cycle arrest in the G2/M phase in CNE-1 and CNE-2 cells. Oxamic acid (0-100 mM; 48 h) induces apoptosis via caspase-3 activation and the mitochondrial pathway in NPC cells. Oxamic acid (0-100 mM; 24 h) increases ROS levels in NPC cells.
Properties of Oxamic acid sodium salt
| Melting point: | 300 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | PBS (pH 7.2): 10 mg/ml |
| form | solid |
| color | White to off-white |
| Water Solubility | Soluble in water (10 mg/ml). |
| BRN | 6538153 |
| InChI | InChI=1S/C2H3NO3.Na/c3-1(4)2(5)6;/h(H2,3,4)(H,5,6);/q;+1/p-1 |
| CAS DataBase Reference | 565-73-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium oxamate (565-73-1) |
Safety information for Oxamic acid sodium salt
Computed Descriptors for Oxamic acid sodium salt
| InChIKey | RQVZIJIQDCGIKI-UHFFFAOYSA-M |
| SMILES | C(=O)(N)C([O-])=O.[Na+] |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 565-73-1 Sodium oxamate 98%View Details
565-73-1 Sodium oxamate 98%View Details
565-73-1 -
 Sodium oxamate 98.00% CAS 565-73-1View Details
Sodium oxamate 98.00% CAS 565-73-1View Details
565-73-1 -
 Sodium oxamate CAS 565-73-1View Details
Sodium oxamate CAS 565-73-1View Details
565-73-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1