Ferric ammonium oxalate trihydrate
Synonym(s):Ammonium ferric oxalate;Iron ammonium oxalate
- CAS NO.:13268-42-3
- Empirical Formula: C2H7FeNO5
- Molecular Weight: 180.93
- MDL number: MFCD00150137
- EINECS: 603-647-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
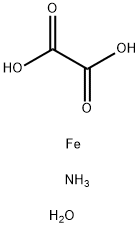
What is Ferric ammonium oxalate trihydrate?
Chemical properties
yellow-green crystals
Chemical properties
Ferric ammonium oxalate is a green crystalline, solid with a granular or salt-like appearance. Color depends on level of iron present
The Uses of Ferric ammonium oxalate trihydrate
Ammonium iron(III) oxalate trihydrate is used in the preparation of Langmuir-Blodgett magnetic thin films. It is also involved in the production of blueprint paper.
General Description
Ammonium ferric oxalate trihydrate decomposes completely at 275°C giving Fe2O3 and has been investigated by thermogravimetry and differential thermal analysis.
Potential Exposure
Ferric ammonium oxalate is used in photography and making blueprints
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from hot water (0.5mL/g). [Beilstein 3 III 1103.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Strong oxidizers may cause release of toxic phosphorus oxides. Organophosphates, in the presence of strong reducing agents such as hydrides, may form highly toxic and flammable phosphine gas. Keep away from alkaline materials.
Properties of Ferric ammonium oxalate trihydrate
| Melting point: | 161°C (rough estimate) |
| Density | 1,78 g/cm3 |
| solubility | very soluble in H2O; insoluble in ethanol |
| form | Crystalline Powder |
| color | Green |
| Water Solubility | Very soluble in water. Insoluble in alcohol |
| Sensitive | Light Sensitive & Hygroscopic |
| Merck | 14,517 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | May decompose upon exposure to light. Incompatible with strong acids, strong oxidizing agents. Hygroscopic. |
| CAS DataBase Reference | 13268-42-3(CAS DataBase Reference) |
| EPA Substance Registry System | Ferric ammonium oxalate (1:3:3) trihydrate (13268-42-3) |
Safety information for Ferric ammonium oxalate trihydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ferric ammonium oxalate trihydrate
Ferric ammonium oxalate trihydrate manufacturer
New Alliance Dye Chem Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 13268-42-3 Ammonium iron(III) oxalate tirydrate 98%View Details
13268-42-3 Ammonium iron(III) oxalate tirydrate 98%View Details
13268-42-3 -
 Ammonium iron(III) oxalate trihydrate CAS 13268-42-3View Details
Ammonium iron(III) oxalate trihydrate CAS 13268-42-3View Details
13268-42-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1