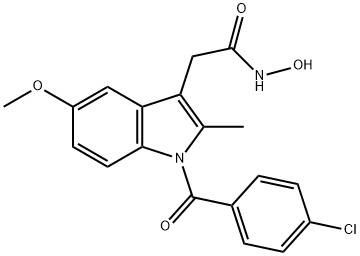
oxametacin
- CAS NO.:27035-30-9
- Empirical Formula: C19H17ClN2O4
- Molecular Weight: 372.806
- MDL number: MFCD00866128
- EINECS: 2481796
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
What is oxametacin ?
Description
Oxametacine is prepared by the acylation of hydroxylamine with indomethacin acid chloride. Oxametacine at a dose of 100 mg is reported to be as effective as 50 mg of indomethacin in reducing pain and inflammation with a lower incidence of adverse effects.
Originator
Flogar, A.B.C. , Italy ,1976
The Uses of oxametacin
Oxametacin is a non-steroidal anti-inflammatory drug.
Definition
ChEBI: A hydroxamic acid obtained by formal condensation of the carboxy group of indometacin with the amino group of hydroxylamine.
Manufacturing Process
1 g of 1-p-chlorobenzoyl-2-methyl-5-methoxy-3-indoleacetic acid [J. Am. Chem. Soc. 85, 488-489 (1963)] is treated in a nitrogen stream with 10 ml thionyl chloride in which it promptly dissolves. The solution is quickly evaporated in vacuum and the residue (which typically is of a deep browngreen color) is distempered, twice or three times, with a few ml anhydrous benzene which is removed in vacuum each time. The resulting residue is thoroughly distempered with 5 ml anhydrous ether which dissolves most of the color impurities, and separated by filtering, purified by crystallizing from plenty of anhydrous ether, yielding a crystalline mass of needles of strawyellow color, melting point 124°C to 127°C. Yield: 0.700 g. Found: Cl % 18.62 (calculated 18.84).
The product is relatively stable towards water and aqueous alkalies in which it proves to be insoluble even after dwelling therein several hours at room temperature. It reacts, better if at elevated temperature, with lower alcohols with which it forms the corresponding esters, and with ammonia under suitable conditions for forming the amide (melting point 219°C to 221°C).
A solution of 1.330 g sodium hydroxide in 20 ml water is slowly admixed with 2.330 g hydroxylamine hydrochloride while cooling, whereupon 1 g chloride of 1-p-chlorobenzoyl-2-methyl-5-methoxy-3-indoleacetic acid is distempered in this neutral or slightly alkaline solution by vigorously stirring during a few minutes.
The acid chloride reacts with the free hydroxylamine with considerable rapidity apparently without dissolving. The reaction is completed when a sample of the suspension shows to become clear on adding aqueous alkali. The crystalline pale-yellow mass of product is separated by filtering, lavishly washed with water and dried in vacuum. The crude product yield is actually quantitative. The product is purified with excellent yields by repeatedly crystallizing from hot dioxane and washing with ether: melting point 181°C to 182°C (dec.).
Therapeutic Function
Antiinflammatory
Trade name
Flogar (UCB, Belgium; ABC, Portugal).
Safety Profile
Poison by ingestion and intraperitoneal routes. An anti-inflammatory agent. When heated to decomposition it emits toxic fumes of Clí and NOx.
Properties of oxametacin
| Melting point: | 181-182° (dec) |
| Density | 1.2671 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| solubility | DMSO (Slightly), Methanol (Heated, Slightly) |
| form | Solid |
| pka | 9.00±0.20(Predicted) |
| color | Crystals from dioxane |
Safety information for oxametacin
Computed Descriptors for oxametacin
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1