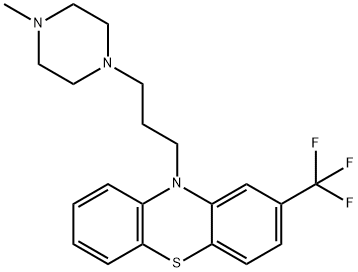
Oxaflumazine
- CAS NO.:16498-21-8
- Empirical Formula: C26H32F3N3O2S
- Molecular Weight: 507.61
- MDL number: MFCD00867754
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:34
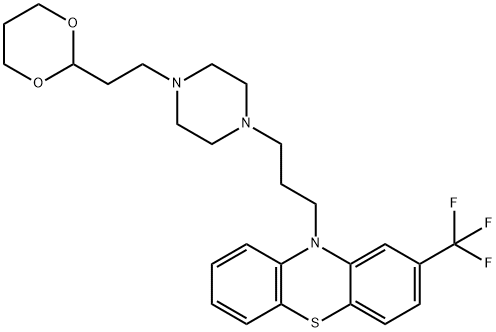
What is Oxaflumazine?
Originator
Oxaflumine, Diamant ,France,1970
Definition
ChEBI: Oxaflumazine is a member of phenothiazines.
Manufacturing Process
Preparation of N-(3-chloropropyl)-N'-[2-(1,3-dioxanyl)-ethyl]-piperazine: A solution of 30 g (0.15 mol) of N-[2-(1,3-dioxanyl)-ethyl]-piperazine and 11.8 g (0.075 mol) of 1-bromo-3-chloropropane in 150 ml of dry benzene was refluxed with stirring for 5 hours. After cooling, the N-[2-(1,3-dioxanyl)ethyl]-piperazinium bromide which had precipitated was filtered off, the filtrate was concentrated in vacuo and the residual oil was distilled. 14.1 g (68%yield) of N-(3-chloropropyl)-N'-[2-1,3-dioxanyl)-ethyl]-piperazine which occurred as a light yellow oil were obtained. Boiling point: 152°C to 155°C under 0.07 mm Hg (nD23 = 1.4940). The disuccinate prepared in acetone and recrystallized from acetone melts at 104°C to 105°C on a hot stage microscope.
The sodium derivative of the 2-trifluoromethylphenothiazine was prepared from 26.7 g (0.1 mol) of 2-trifluoromethylphenothiazine and 2.3 g (0.1 g atom) of sodium in 500 ml of liquid ammonia. After the reaction was completed, the ammonia was driven off and 500 ml of dry toluene were added. A solution of 25 g (0.09 mol) of N-(3-chloropropyl)-N'-[2-(1,3dioxanyl)-ethyl]-piperazine in 200 ml of toluene was added drop by drop to this solution which was then refluxed with stirring for 18 hours. After cooling, the precipitate which had formed was filtered and the filtrate was washed with water, dried and concentrated in vacuo. 33 g of brown oil, the N-3-(2trifluoromethyl-10-phenothiazinyl)-propyl-N'-2-[2-(1,3-dioxanyl)]-ethylpiperazine, were obtained.
A warm solution of 4.4 g of the base obtained in 100 ml of acetonitrile was added to a warm solution of succinic acid in 200 ml of acetonitrile. After standing for 15 hours at 0°C. the crystalline product was obtained, melting point 138°C.
Therapeutic Function
Neuroleptic, Antihistaminic, Spasmolytic
Properties of Oxaflumazine
| Boiling point: | 605.5±55.0 °C(Predicted) |
| Density | 1.235±0.06 g/cm3(Predicted) |
| pka | 7.65±0.10(Predicted) |
Safety information for Oxaflumazine
Computed Descriptors for Oxaflumazine
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole 4-Ethylbenzylamine N-(5-Amino-2-methylphenyl)acetamide 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazoleRelated products of tetrahydrofuran

You may like
-
 100-71-0 99%View Details
100-71-0 99%View Details
100-71-0 -
 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
127464-43-1 -
 13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 -
 1446013-08-6 98%View Details
1446013-08-6 98%View Details
1446013-08-6 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3