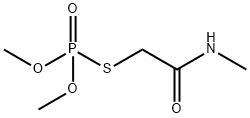
Omethoate
- CAS NO.:1113-02-6
- Empirical Formula: C5H12NO4PS
- Molecular Weight: 213.19
- MDL number: MFCD00055441
- EINECS: 214-197-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:33
What is Omethoate?
The Uses of Omethoate
Omethoate is a systemic insecticide used for the control of (mostly) sucking insects and mites in a wide variety of crops.
The Uses of Omethoate
Insecticidal, acaricidal, and fungicidal combinations of carboxamides and other pesticides.
What are the applications of Application
Omethoate is an insecticidal, acaricidal, and fungicidal combination of carboxamides and other pesticides
Definition
ChEBI: Omethoate is an organic thiophosphate and an organothiophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide and an agrochemical. It is functionally related to a N-methyl-2-sulfanylacetamide.
Contact allergens
Contact dermatitis from omethoate-dimethoxon is rare.
Metabolic pathway
Omethoate is the P=O analogue (oxon) of dimethoate. Dimethoate is metabolised via oxidative desulfuration to omethoate which is the active anti-acetylcholinesterase metabolite. This biotransformation occurs in all media: hence the metabolic pathways of both compounds have much in common. However, degradative pathways acting directly on dimethoate such as O-demethylation, N-demethylation and hydrolysis of the amide function mean that the balance between activation and degradative metabolism will influence their respective selective toxicities. In mammals there are two main routes of degradation: (i) glutathione transferase mediated O-demethylation (ii) hydrolysis to N-methylthioglycolamide which is S-methylated by S-adenosylmethionine and subsequently thiooxidised. In soil and plants the N-methylthioglycolamide moiety formed from the hydrolysis of omethoate undergoes a complex series of C1, C2 and C3 biotransformations ultimately leading to oxalate and citrate respectively and total degradation to C02. The information reported below is derived largely from an evaluation prepared by the UK MAFF Pesticide Safety Directorate (PSD, 1993).
Metabolism
Orally administered omethoate to rats is rapidly metabolized and excreted in the urine; the main metabolites are O-demethylomethoate and N-methyl-2- methylsulfinylacetamide. O-Demethylation and hydrolysis of the P?S bond are main degradation routes both in mammals and plants. Omethoate is rapidly degraded in soils with DT50 of a few days.
Degradation
Omethoate was slowly hydrolysed in acidic media but more rapidly under alkaline conditions. The half-lives for hydrolysis at pH 4,7, and 9 were 102 days, 17 days and 28 hours, respectively (PM). Omethoate does not absorb light at wavelengths above 250 nm and it is thus unlikely to be subject to photodecomposition. An aqueous solution of omethoate was irradiated for 14 hours with a high pressure filtered mercury vapour lamp without detectable photolysis occurring (PSD, 1993).
Toxicity evaluation
The acute oral LD50 for rats is about 25 mg/kg. Inhalation LC50 (4 h) for rats is 0.3 mg/L air. ADI is 0.3 μg/kg b.w.
Properties of Omethoate
| Melting point: | -27.9°C |
| Boiling point: | 135°C |
| Density | 1.3200 |
| vapor pressure | 3.3×10-3Pa (20 °C) |
| Flash point: | 100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | 14.40±0.46(Predicted) |
| form | liquid |
| Water Solubility | Miscible |
| BRN | 1785256 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 1113-02-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Omethoate(1113-02-6) |
| EPA Substance Registry System | Omethoate (1113-02-6) |
Safety information for Omethoate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H311:Acute toxicity,dermal H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Omethoate
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Omethoate CAS 1113-02-6View Details
Omethoate CAS 1113-02-6View Details
1113-02-6 -
 Omethoate CAS 1113-02-6View Details
Omethoate CAS 1113-02-6View Details
1113-02-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4