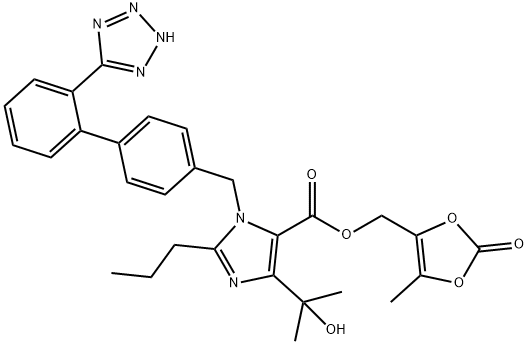
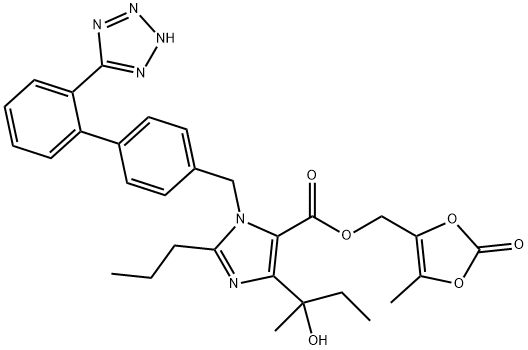
Olmesartan Medoxomil
Synonym(s):4-(1-Hydroxy-1-methylethyl)-2-propyl-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-carboxylic acid (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester;CS-866;Olmesartan medoxomil
- CAS NO.:144689-63-4
- Empirical Formula: C29H30N6O6
- Molecular Weight: 558.59
- MDL number: MFCD00944911
- EINECS: 604-433-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Olmesartan Medoxomil?
Description
Olmesartan medoxomil (Olmetec, Benicar) is an angiotensin II type 1 (AT(1)) receptor antagonist (angiotensin receptor blocker [ARB]) that inhibits the actions of angiotensin II on the renin-angiotensin-aldosterone system, which plays a key role in the pathogenesis of hypertension. It works by blocking a substance in the body that causes the blood vessels to tighten. As a result, olmesartan relaxes the blood vessels. This lowers blood pressure and increases the supply of blood and oxygen to the heart.
Chemical properties
White to off-white crystalline powder
Originator
Sanky (Japan)
The Uses of Olmesartan Medoxomil
Olmesartan medoxomil is used to treat high blood pressure (hypertension). Lowering high blood pressure helps prevent strokes, heart attacks, and kidney problems.
Definition
ChEBI: Olmesartan medoxomil is a member of biphenyls.
brand name
Benicar
General Description
Olmesartan Medoxomil is a synthetic imidazole derivative prodrug with an antihypertensive property. Upon hydrolysis, olmesartan medoxomil is converted to olmesartan. Olmesartan selectively binds to the angiotensin type 1 (AT1) receptor of angiotensin II in vascular smooth muscle and adrenal gland, thereby competing angiotensin II binding to the receptor. This prevents angiotensin II-induced vasoconstriction and decreases aldosterone production, thereby preventing aldosterone-stimulated sodium retention and potassium excretion.
Biochem/physiol Actions
Olmesartan medoxomil is a selective Angiotensin II Type I receptor blocker and antihypertensive drug. Olmesartan medoxomil is converted enzymatically to the active form olmesartan.
Clinical Use
Angiotensin-II receptor antagonist:
Hypertension
Side Effects
Dizziness or lightheadedness may occur as your body adjusts to the medication. If any of these effects persist or worsen, tell your doctor or pharmacist promptly. To reduce the risk of dizziness and lightheadedness, get up slowly when rising from a sitting or lying position.
Synthesis
Olmesartan Medoxomil can be synthesized in 8 steps from diaminomaleonitrile by successive reactions with trialkylorthopropanoate to access 2-propyl-imidazole-45dicarbonitrile, conversion of the two nitrile functions to the corresponding ethyl esters, followed by methylmagnesium bromide addition to give the corresponding 4-(1-hydroxyalkyl)imidazole derivative. 
The imidazole ring of olmesartan (18) was constructed with diaminomaleonitrile 155 and trimethylorthobutyrate (156) in CH3CN then xylene to give 157 in 96% yield. Acid hydrolysis of 157 in 6N HCl gave the dicarboxylic acid intermediate. After esterification of the diacid in ethanol in the presence of HCl, diester 158 was treated with MeMgCl to give 4-(1-hydroxyalkyl) imidazole 159 in 95% yield. Alkylation of 159 with biphenyl bromide 160 in the presence of potassium tbutoxide afforded 161 in 80% yield. Ester 161 was then hydrolyzed to free carboxylic acid 162 under basic conditions, and 162 was treated with chloride 163 in the presence of K2CO3 to give ester 164 in 88% yield from 161.Lastly, the trityl group was removed with 25% aqueous acetic acid to give olmesartan (18) in 81% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia
hypotension and renal impairment with ACE-Is and
aliskiren.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Lithium: reduced excretion (possibility of enhanced
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity.
Side Effects
The more common side effects that occur with olmesartan include:
back pain
bronchitis
diarrhea
headache
blood in your urine
high blood sugar
high triglycerides
flu-like symptoms, such as fever and body aches
sore throat, runny nose, and sinus infection
Metabolism
Olmesartan medoxomil is an ester prodrug that is hydrolysed during absorption from the gastrointestinal tract to the active form olmesartan. It is excreted in the urine and the bile as olmesartan; about 35-50% of the absorbed dose is excreted in the urine and the remainder in the bile.
Properties of Olmesartan Medoxomil
| Melting point: | 180°C |
| Boiling point: | 804.2±75.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| Flash point: | 180°C |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 4.15±0.10(Predicted) |
| color | white to beige |
| Decomposition | 180 ºC |
| CAS DataBase Reference | 144689-63-4(CAS DataBase Reference) |
Safety information for Olmesartan Medoxomil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Olmesartan Medoxomil
| InChIKey | UQGKUQLKSCSZGY-UHFFFAOYSA-N |
| SMILES | C1(CCC)N(CC2=CC=C(C3=CC=CC=C3C3=NNN=N3)C=C2)C(C(OCC2=C(C)OC(=O)O2)=O)=C(C(O)(C)C)N=1 |
Olmesartan Medoxomil manufacturer
CEFA CILINAS BIOTICS PVT LTD
Gemini Exports
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![1-(4-(2-hydroxypropan-2-yl)-2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazol-5-yl)ethanone](https://img.chemicalbook.in/CAS/20180531/GIF/1227626-50-7.gif)
You may like
-
 144689-63-4 98%View Details
144689-63-4 98%View Details
144689-63-4 -
 Olmesartan Medoxomil 97%View Details
Olmesartan Medoxomil 97%View Details -
 Olmesartan medoxomil 98%View Details
Olmesartan medoxomil 98%View Details -
 144689-63-4 Olmesartan medoxomil 98%View Details
144689-63-4 Olmesartan medoxomil 98%View Details
144689-63-4 -
 144689-63-4 Olmesartan medoxomil 98%View Details
144689-63-4 Olmesartan medoxomil 98%View Details
144689-63-4 -
 Olmesartan medoxomil >98% (HPLC) CAS 144689-63-4View Details
Olmesartan medoxomil >98% (HPLC) CAS 144689-63-4View Details
144689-63-4 -
 Olmesartan medoxomil 95.00% CAS 144689-63-4View Details
Olmesartan medoxomil 95.00% CAS 144689-63-4View Details
144689-63-4 -
 Olmesartan Medoxomil Powder API, IPView Details
Olmesartan Medoxomil Powder API, IPView Details
144689-63-4
