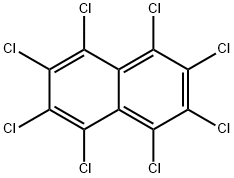
Octachloronaphthalene
- CAS NO.:2234-13-1
- Empirical Formula: C10Cl8
- Molecular Weight: 403.73
- MDL number: MFCD00145026
- EINECS: 218-778-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is Octachloronaphthalene?
Chemical properties
The chlorinated naphthalenes in which one or more hydrogen atoms have been replaced by chlorine to form wax-like substances, beginning with monochloronaphthalene and going on to the octachlor derivatives. Their physical states vary from mobile liquids to waxysolids depending on the degree of chlorination; freezing/ melting points of the pure compounds range from 17C for 1-chloronaphthalene to 198C for 1,2,3,4- tetrachloronaphthalene. 1-Chloro-isomer: Hazard identification (based on NFPA-704 M Rating System): Health 2, flammability 1, reactivity 0. 2-Chloro-isomer:
The Uses of Octachloronaphthalene
Chemical research; organic synthesis. According to HSDB (1005), U.S. has discontinued manufacturing of chlorinated naphthalenes since 1977. Chlorinated naphthalenes were formerly used as a wood preservative, additives in cutting oils, and as an additive in fireproofing and waterproofing cable insulation.
The Uses of Octachloronaphthalene
In electric cable insulation; additive to lubricants
The Uses of Octachloronaphthalene
Octachloronapthalene is a polychlorinated napththalene (PCN) produced via industrial thermal processes. It is a chemical pollutant present in the environment.
General Description
Pale yellow solid with an aromatic odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Contact with strong oxidizing agents may cause fire and explosion .
Health Hazard
The higher-chlorinated naphthalenes may cause severe injury to the liver.
Fire Hazard
Literature sources indicate that Octachloronaphthalene is nonflammable.
Safety Profile
Poison by inhalation, ingestion, and skin contact. When heated to decomposition it emits highly toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC.
Potential Exposure
Industrial exposure from individual chlorinated naphthalenes is rarely encountered; rather it usually occurs from mixtures of two or more Chlorinated naphthalenes. Due to their stability, thermoplasticity, and nonflammability, these compounds enjoy wide industrial application. These compounds are used in the production of electric condensers; in the insulation of electric cables and wires; as additives to extreme pressure lubricants; as supports for storage batteries; and as a coating in foundry use. octachloro-: Used as a fireproof and waterproof additive and lubricant additive. Pentachloro-: Used in electric wire insulation and in additives to special lubricants. tetrachloro-: Used in electrical insulating materials and as an additive in cutting oils. trichloro-: Used in lubricants and in the manufacture of insulation for electrical wire. Because of the possible potentiation of the toxicity of higher Chlorinated naphthalenes by ethanol and carbon tetrachloride, individuals who ingest enough alcohol to result in liver dysfunction would be a special group at risk. Individuals, e.g., analytical and synthetic chemists, mechanics and cleaners, who are routinely exposed to carbon tetrachloride or other hepatotoxic chemicals would also be at a greater risk than a population without such exposure. Individuals involved in the manufacture, utilization, or disposal of polychlorinated naphthalenes would be expected to have higher levels of exposure than the general population.
Source
Octachloronaphthalene was detected in various technical-grade PCB mixtures as impurities or by-products. Concentrations were 0.098 μg/g in PCB-1016, 0.433 μg/g in PCB- 1232, 0.378 μg/g in PCB-1248, 3.23 μg/g in PCB-1254, 55.5 μg/g in PCB-1260, and 50.5 μg/g in PCB-1262 (Yamashita et al., 2000).
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
All are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Keep away from heat. Penta- is also incompatible with acids, alkalis.
Waste Disposal
High-temperature incineration with flue gas scrubbing. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of Octachloronaphthalene
| Melting point: | 185°C |
| Boiling point: | 440°C |
| Density | 2.00 |
| vapor pressure | 4.20 x 10-7 mmHg at 25.00 °C (Lei et al., 1999) |
| refractive index | 1.6000 (estimate) |
| Flash point: | -18 °C |
| storage temp. | APPROX 4°C
|
| solubility | Soluble in benzene, chloroform, and ligroin (Weast, 1986) |
| form | neat |
| color | Waxy, light yellow solid with an aromatic odor |
| Water Solubility | Insoluble |
| BRN | 1653604 |
| Exposure limits | NIOSH REL: TWA 0.1, STEL 0.3; OSHA PEL: TWA 0.1; ACGIH
TLV: TWA 0.1, STEL, 0,3 (adopted). |
| CAS DataBase Reference | 2234-13-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, octachloro-(2234-13-1) |
| EPA Substance Registry System | Octachloronaphthalene (2234-13-1) |
Safety information for Octachloronaphthalene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Octachloronaphthalene
| InChIKey | RTNLUFLDZOAXIC-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1