o-Toluidine
Synonym(s):2-Aminotoluene;2-Aminotoluene, 2-Methylaniline;2-Methylaniline;o-Toluidine
- CAS NO.:95-53-4
- Empirical Formula: C7H9N
- Molecular Weight: 107.15
- MDL number: MFCD00007730
- EINECS: 202-429-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
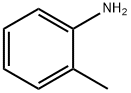
What is o-Toluidine?
Chemical properties
Light-yellow liquid, becomes reddishbrown on exposure to air and light. Soluble in alcohol and ether; very slightly soluble in water. Combustible.
Chemical properties
O-Toluidine is a light yellow to reddish brown liquid. On exposure to light and atmospheric air, the compound quickly turns a dark color. The compound has extensive use in a large number of industries around the world. For instance, as an intermediate in the manufacture of azo and indigo dyes, pigments, sulfur dyes, pesticides, pharmaceutical products, rubber and vulcanizing chemicals, and similar products.
Chemical properties
o-Toluidine is a colorless to pale yellow liquid with a weak, pleasant, aromatic odor.
Physical properties
Colorless to pale yellow liquid with an aromatic, aniline-like odor. Becomes reddish-brown on exposure to air and light. Odor threshold concentration is 250 ppb (quoted, Amoore and Hautala, 1983).
Occurrence
O-Toluidine has been reported to be a component of tar produced by low-temperature carbonization of coal and has been detected in gasoline fractions from arlan petroleum, tobacco leaf extracts, and in the aroma components of black tea (Anon. 1972). Patrianakos and Hoffmann (1979) detected it in tobacco smoke. It is a metabolite of prilocaine anesthetic in rats (Akerman et al 1966) and humans (Hjelm et al 1972; Struck et al 1969). It is a possible contaminant of bootleg methaqualone (Goldfarb and Finelli 1974) but is not a methaqualone metabolite (Nowak et al 1966). It is also a metabolite of the dye Poncean 3R (Lindstrom et al 1969).O-Toluidine has been detected as a contaminant in injectables stored in polystyrene (Ahmad, 1982).
The Uses of o-Toluidine
o-Toluidine is used in the manufacture ofvarious dyes, in printing textiles blue-blackand as an intermediate in rubber chemicals,pesticides, and pharmaceuticals.
The Uses of o-Toluidine
It was used in determination of glucose in biological materials.It was used as precursor in the synthesis of poly(o-toluidine) and copper nanoparticle composite material.
The Uses of o-Toluidine
A carcinogenic and toxic aromatic amine contained in hair dye, henna and dyed hair samples.
o-Toluidine, is a methemoglobin-inducing chemical and a human carcinogen. Commercial production of o-toluidine was first reported in the United States in 1922. o-Toluidine and its hydrochloride salt are used primarily as intermediates in the manufacture of dyes and pigments for printing textiles, in color photography, and as biologic stains. In addition, o-toluidine is used as an intermediate for rubber vulcanizing chemicals, pharmaceuticals, and pesticides. Other minor uses include intermediate for organic synthesis and clinical laboratory reagent for glucose analysis (IARC, 2000, 2010; Woo and Lai, 2012).
Production Methods
The production of O-toluidine is based on the catalytic hydrogenation of O-nitrotoluene or the amination of toluene with methylhydroxylamine in the presence of aluminum trichloride (Windholz 1983). It is available as a technical grade with a minimum of 99.5% purity containing m-toluidine (0.4% maximum) and/or ptoluidine (0.1% maximum) as impurities (Anon. 1978). The stabilized technical grade may also contain less than 0.5% of unidentified stabilizing agents to prevent darkening.
Definition
ChEBI: An aminotoluene in which the amino substituent is ortho to the methyl group.
Synthesis Reference(s)
Journal of the American Chemical Society, 113, p. 1054, 1991 DOI: 10.1021/ja00003a056
The Journal of Organic Chemistry, 43, p. 731, 1978 DOI: 10.1021/jo00398a046
Chemical and Pharmaceutical Bulletin, 34, p. 3905, 1986 DOI: 10.1248/cpb.34.3905
General Description
A clear colorless or light yellow liquid. May become reddish brown on exposure to air and light. Flash point 185°F. Has about the same density as water and is very slightly soluble in water. Vapors are heavier than air. Confirmed carcinogen.
Air & Water Reactions
Becomes reddish brown upon exposure to air and light [Hawley]. Slightly soluble in water.
Reactivity Profile
o-Toluidine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Emits very toxic oxides of nitrogen when heated to decomposition. Undergoes a hypergolic reaction with red fuming nitric acid [Kit and Evered, 1960, p. 239, 242].
Health Hazard
Absorption of toxic quantities by any route causes cyanosis (blue discoloration of lips, nails, skin); nausea, vomiting, and coma may follow. Repeated inhalation of low concentrations may cause pallor, low-grade secondary anemia, fatigability, and loss of appetite. Contact with eyes causes irritation.
Health Hazard
Exposures to O-toluidine cause toxicity and poisoning to animals and occupational workers. It is highly toxic to animals and humans and is rapidly absorbed by oral, dermal, and inhalation by mammals. The acute oral LD50 to rats ranges from 900 to 940 mg/kg. The compound is known to cause adverse effects in workers, which include headache, irritation of skin, eye, kidneys, bladder, and hematuria. O-Toluidine has caused hepatocellular adenoma and carcinoma in experimental laboratory mice and rats. Occupational workers exposed to O-toluidine have also demonstrated bladder cancer although the role of aniline cannot be ruled out. However, the IARC working group, because of insuffi cient data, classify O-toluidine as a Group 2B agent, meaning possibly carcinogenic to humans, while the NIOSH classify this compound as an occupational carcinogen, and the ACGIH label it as a suspected human carcinogen under A2 class.
Health Hazard
o-Toluidine is a cancer-causing compound.Its acute toxicity in test species was lowto moderate. Severe poisoning may occur athigh doses. It may enter the body by inhala tion of its vapors, ingestion, or absorptionthrough skin contact. The target organs arekidneys, liver, blood, cardiovascular system,skin, and eyes. The toxic symptoms includemethemoglobinemia, anemia, and reticulo cytosis, which are similar to aniline. Thisproduces anoxia (lack of oxygen), cyanosis,headache, weakness, drowsiness, dizziness,increase in urine volume, and hematuria.The pure liquid on skin contact can causeirritation and dermatitis. Contact with eyescan cause burns.
LD50 value, oral (mice): 520 mg/kg
o-Toluidine is a suspected human carcinogen. The evidence of carcinogenicity inhuman is inadequate. It caused tumors in thekidney, bladder, and lungs in rats, mice, andrabbits resulting from oral and subcutaneousadministration.
Brennan and Schiestl (1999) have reportedo-toluidine and o-anisidine induced free radicals and intrachromosomal recombination inSaccharomyces cerevisiae. The toxicity andrecombination induced by these compoundswere reduced by free radical scavenger andantioxidant N-acetyl cysteine.
Health Hazard
Acute O-toluidine poisoning can cause painful hematuria (Goldbarb and Finelli, 1974) and methemoglobinemia (Hjelm et al 1972 and Struck et al 1969) in humans. Conflicting reports have resulted from evaluating the role of the compound in causing bladder or other cancers. Olt and Langner (1983) reported no increase in men engaged in the production of organic dyes, but Rubino et al (1982) strongly implicated O-toluidine in deaths due to bladder cancer in similar types of men.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen and flammable vapors may form in fire.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
O-Toluidine is used as an intermediate of the manufacturing of triphenylmethane dyes and safranine colors (Northcott 1978; Windholz 1983). It is an antioxidant in rubber manufacturing and a pharmaceutical intermediate. It has been used as a laboratory reagent in glucose analysis and also used in the preparation of ion exchange resins and making various colors fast to acid.
Safety Profile
Confirmed carcinogen with experimental neoplastigenic and tumorigenic data. Poison by ingestion and intraperitoneal routes. Moderately toxic by skin contact. Human systemic effects by inhalation: urine volume increase, hematuria, and blood methemoglobinemiacarboxyhemoglobinemia. An experimental teratogen. Human mutation data reported. A skin and severe eye irritant. Human mucous membrane effects. Can produce severe systemic disturbances. The main portal of entry into the body is the respiratory tract, particularly in cases of industrial exposure. The symptoms produced are headache, weakness, difficulty in breathing, air hunger, psychic dsturbances, and marked irritation of the kidneys and bladder. The literature does not yield any good data for comparing the toxicity of the o-, m-, and p-isomers. Their behavior is generally comparable to that of aniline. It has been determined experimentally that a concentration of about 100 ppm is the maximum endurable for 1 hour without serious consequences and that 6-23 ppm is endurable for several hours without serious disturbances. Flammable when exposed to heat or flame. Hypergolic reaction with red fuming nitric acid. Can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of NOx. See also ANILINE.
Potential Exposure
o-Toluidine is used as an intermediate in the manufacture of dyes; as an intermediate in pharmaceutical manufacture; in textile printing; in rubber accelerators; in production of o-aminoazotoluene
Carcinogenicity
o-Toluidine and o-toluidine hydrochloride are reasonably anticipatedto be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals.
Source
As o+p-toluidine, detected in distilled water-soluble fractions of 87 octane gasoline and Gasohol at concentrations of 0.80 and 0.19 mg/L, respectively (Potter, 1996). o-Toluidine was detected in 77% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from Boston, MA). At 25 °C, concentrations ranged from 13 to 18,000 μg/L in gasoline and 10 to 1,400 μg/L in water-soluble fractions. Average concentrations were 6.1 mg/L in gasoline and 0.47 mg/L in water-soluble fractions (Schmidt et al., 2002).
Environmental Fate
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.40 g/g which is 55.1%
of the ThOD value of 2.54 g/g.
Chemical/Physical. Kanno et al. (1982) studied the aqueous reaction of o-toluidine and other
substituted aromatic hydrocarbons (aniline, toluidine, 1- and 2-naphthylamine, phenol, cresol,
pyrocatechol, resorcinol, hydroquinone, and 1-naphthol) with hypochlorous acid in the presence of
ammonium ion. They reported that the aromatic ring was not chlorinated as expected but was
cleaved by chloramine forming cyanogen chloride. As the pH was lowered, the amount of
cyanogen chloride formed increased (Kanno et al., 1982).
o-Toluidine will not hydrolyze because it does not contain a hydrolyzable functional group
(Kollig, 1993).
Metabolism
Absorption of O-toluidine from the gastrointestinal tract in rats is rapid with peak blood values at 1 h; blood values were near zero in 24 h (Sencyuk and Rucinska 1984a). The urine was the main excretory route; > 92% in 24 h (Cheever et al 1980). At an oral dose of 20 mg/kg, 26% was excreted in the urine in 24 h as O-toluidine (Senczuk and Rucinska 1984b). Kulkarni et al (1983) demonstrated that N-hydroxy-O-toluidine and O-nitrosotoluene are urinary metabolites of otoluidine. Other urinary metabolites in rats have included conjugated aminomethylphenols (Cheever et al 1980), azoxytoluene, N-acetyl-O-toluidine, N-acetyl-oaminobenzylalcohol, 4-amino-m-cresol, N-acetyl-4-amino-m-cresol, anthranilic acid, N-acetylanthranilic acid (Son et al 1980). Sulfate conjugates predominate over glucuronides by a ratio of 6:1. Thus, in rats, the major metabolic routes are N-acetylation and 4-hydroxylation. Human urinary metabolites (after administration of prilocaine) included O-toluidine,P-hydroxy-O-toluidine, and O-hydroxy-toluidine (Hjelm et al 1972). The primary metabolism of O-toluidine takes place in the endoplasmic reticulum. Exposure to O-toluidine enhances the microsomal activity of aryl hydrocarbon hydroxylase (particularly in kidney), NADPH-cyto-chrome c reductase and the content of cytochrome P-450 (Gnojkowski et al 1984).
Shipping
UN1708 Toluidines, liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
In general, methods similar to those for purifying aniline can be used, e.g. distillation from zinc dust, at reduced pressure, under nitrogen. Berliner and May [J Am Chem Soc 49 1007 1927] purified it via the oxalate. Twice-distilled o-toluidine is dissolved in four times its volume of diethyl ether, and the equivalent amount of oxalic acid needed to form the dioxalate is added as its solution in diethyl ether. (If p-toluidine is present, its oxalate precipitates and can be removed by filtration.) Evaporation of the ethereal solution gives crystals of o-toluidine dioxalate [Beilstein 12 III 1494, 12 IV 1817]. These are filtered off, recrystallised five times from water containing a small amount of oxalic acid (to prevent hydrolysis), then treated with dilute aqueous Na2CO3 to liberate the amine which is separated, dried (CaCl2) and distilled under reduced pressure. The benzoyl derivative has m 144o (from EtOH). [Beilstein 12 H 772, 12 I 372, 12 II 429, 12 III 1837, 12 IV 1744.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices).
Properties of o-Toluidine
| Melting point: | -23 °C |
| Boiling point: | 199-200 °C(lit.) |
| Density | 1.008 g/mL at 25 °C(lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 0.26 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 185 °F |
| storage temp. | 2-8°C |
| solubility | 1.5 g/100 mL (25°C) |
| form | liquid |
| Colour Index | 37077 |
| pka | 4.44(at 25℃) |
| color | light yellow to light amber |
| Odor | Aromatic, aniline-like. |
| PH | 7.4 (H2O, 20℃)Aqueous solution |
| explosive limit | 1.5-7.5%(V) |
| Water Solubility | 1.5 g/100 mL (25 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,9536 |
| BRN | 741981 |
| Henry's Law Constant | 1.98 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | TLV-TWA 2 ppm (~9 mg/m3) (ACGIH),
5 ppm (~22 mg/m3) (MSHA, OSHA, and
NIOSH); IDLH level 100 ppm (NIOSH);
carcinogenicity: Suspected Human Carcino gen (ACGIH), Human Limited Evidence
(IARC). |
| Dielectric constant | 6.3399999999999999 |
| CAS DataBase Reference | 95-53-4(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 77, 99, 100F) 2012 |
| NIST Chemistry Reference | Benzenamine, 2-methyl-(95-53-4) |
| EPA Substance Registry System | o-Toluidine (95-53-4) |
Safety information for o-Toluidine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for o-Toluidine
| InChIKey | RNVCVTLRINQCPJ-UHFFFAOYSA-N |
o-Toluidine manufacturer
JSK Chemicals
Clickchem Research LLP
CEFA CILINAS BIOTICS PVT LTD
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 o-Toluidine 98%View Details
o-Toluidine 98%View Details -
 o-Toluidine 98%View Details
o-Toluidine 98%View Details -
 o-Tolidine reagent CAS 95-53-4View Details
o-Tolidine reagent CAS 95-53-4View Details
95-53-4 -
 Total Hardness Indicator Tablets CAS 95-53-4View Details
Total Hardness Indicator Tablets CAS 95-53-4View Details
95-53-4 -
 Ortho Toluidine CASView Details
Ortho Toluidine CASView Details -
 o-Toluidine reagent CASView Details
o-Toluidine reagent CASView Details -
 99.5% Industrial Grade Ortho Toluidine CAS 95-53-4, 50 kgView Details
99.5% Industrial Grade Ortho Toluidine CAS 95-53-4, 50 kgView Details
95-53-4 -
 O- Toluidine chemical, 95-53-4, Grade: TechnicalView Details
O- Toluidine chemical, 95-53-4, Grade: TechnicalView Details
95-53-4
