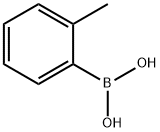
o-Toluic acid
Synonym(s):2-Methylbenzoic acid
- CAS NO.:118-90-1
- Empirical Formula: C8H8O2
- Molecular Weight: 136.15
- MDL number: MFCD00002477
- EINECS: 204-284-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
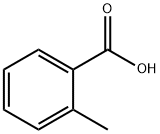
What is o-Toluic acid?
Chemical properties
White crystals. Slightly soluble in water; soluble in alcohol and chloroform. Combustible.
The Uses of o-Toluic acid
o-Toluic acid is part of a group of Benzoic acid (B203900) derivatives that possess inhibitory activity against mushroom tyrosinases. o-Toluic acid is also used as a reagent to synthesize halogen-substituted benzoic acids (such as 2,4-Dichlorobenzoic acid [D431955]), which have potential muscle stimulating effects.
The Uses of o-Toluic acid
Bacteriostat.
Definition
ChEBI: A methylbenzoic acid that is benzoic acid substituted by a methyl group at position 2.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 2, p. 588, 1943
The Journal of Organic Chemistry, 25, p. 616, 1960 DOI: 10.1021/jo01074a035
Tetrahedron Letters, 22, p. 1013, 1981 DOI: 10.1016/S0040-4039(01)82853-7
Air & Water Reactions
Fine dust dispensed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard. . Insoluble in water.
Reactivity Profile
o-Toluic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in o-Toluic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. o-Toluic acid is incompatible with strong oxidizers.
Fire Hazard
Flash point data for o-Toluic acid are not available. o-Toluic acid is probably combustible. Fine dust dispensed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard.
Purification Methods
Crystallise the acid from *benzene (2.5mL/g) and dry in air. The S-benzylisothiuronium salt has m 146o (from aqueous EtOH). [Beilstein 9 IV 1697.]
Properties of o-Toluic acid
| Melting point: | 102-104 °C (lit.) |
| Boiling point: | 258-259 °C (lit.) |
| Density | 1.062 g/mL at 25 °C (lit.) |
| refractive index | 1.512 |
| Flash point: | 148 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 1.2g/l |
| form | Needle-Like Crystalline Solid |
| pka | 3.91(at 25℃) |
| color | White to slightly yellow |
| Specific Gravity | 1.062 |
| PH | 3.1 (H2O, 20℃)(saturated aqueous solution) |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Merck | 14,9535 |
| BRN | 1072103 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 118-90-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 2-methyl-(118-90-1) |
| EPA Substance Registry System | o-Toluic acid (118-90-1) |
Safety information for o-Toluic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for o-Toluic acid
| InChIKey | ZWLPBLYKEWSWPD-UHFFFAOYSA-N |
o-Toluic acid manufacturer
Kaival Chemicals Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 o-Toluic Acid pure CAS 118-90-1View Details
o-Toluic Acid pure CAS 118-90-1View Details
118-90-1 -
 2-Toluic acid CAS 118-90-1View Details
2-Toluic acid CAS 118-90-1View Details
118-90-1 -
 O-Toluic Acid CASView Details
O-Toluic Acid CASView Details -
 o-Toluic Acid CAS 118-90-1View Details
o-Toluic Acid CAS 118-90-1View Details
118-90-1 -
 o-Toluic acid CAS 118-90-1View Details
o-Toluic acid CAS 118-90-1View Details
118-90-1 -
 Powder Ortho Toluic Acid(2-Methyl Benzoic Acid), Packaging Type: LLDPE Bag, Packaging Size: 40 KgView Details
Powder Ortho Toluic Acid(2-Methyl Benzoic Acid), Packaging Type: LLDPE Bag, Packaging Size: 40 KgView Details
118-90-1 -
 O-Toluic Acid Powder, 99%View Details
O-Toluic Acid Powder, 99%View Details
118-90-1 -
 2-METHYLBENZOIC ACID (118-90-1), PowderView Details
2-METHYLBENZOIC ACID (118-90-1), PowderView Details
118-90-1
