o-Phenanthroline
- CAS NO.:66-71-7
- Empirical Formula: C12H8N2
- Molecular Weight: 180.21
- MDL number: MFCD00011678
- EINECS: 200-629-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
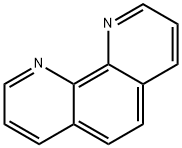
What is o-Phenanthroline?
Description
o-Phenanthroline is an organic intermediate, white crystalline powder. It is a redox indicator, a reagent for the determination of ferrous, palladium, vanadium, copper and iron.
Description
1,10-Phenanthroline forms a stable complex with Fe(II) ion called ferroin, which is used as an indicator in Fe(II) salt titrations. Ferroin is also used in the determination of other metals, such as nickel, ruthenium, and silver. Phenanthroline is synthesized by heating?o-phenylenediamine or 8-aminoquinoline with glycerol, H2SO4, and nitrobenzene.
Chemical properties
Monohydrate is a white crystalline powder. The melting point is 93-94°C, the melting point of anhydrous is 117°C, soluble in 300 parts of water, 70 parts of benzene, soluble in alcohol and acetone.
Characteristics
o-Phenanthroline can form complexes with a variety of transition metals. Since the complexes formed are chelates, they are relatively stable. The complexes and their derivatives formed with copper can be used as non-oxidative nucleic acid cleaving enzymes because they have certain cleavage activity on DNA, and further have certain anticancer activities.
The Uses of o-Phenanthroline
A chelating agent, forming complexes with most metal ions.
The Uses of o-Phenanthroline
As an analytical reagent for determination of metals in chemical and biological systems through complex formation. As an indicator ("Ferroin") in combination with ferrous ions for oxidation/reduction reactions. In organic syntheses as an activator.
What are the applications of Application
1,10-Phenanthroline is a ligand employed in the spectrophotometric determination of metals and photocatalytic reduction of carbon dioxide
Definition
ChEBI: 1,10-phenanthroline is a phenanthroline. It has a role as an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor and an EC 2.7.1.1 (hexokinase) inhibitor.
Preparation
1,10-Phenanthroline is prepared by heating o-phenylenediamine, glycerin, nitrobenzene and concentrated sulfuric acid.
Safety Profile
Poison by ingestion and intraperitoneal routes. An experimental teratogen. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
in vitro
proteolysis of lamins, which are the intermediate filament proteins providing support to the nuclear structural proteins and are targets of mmp-2, o-phenanthroline abolished the proteolysis of lamin a [2].
storage
Desiccate at RT
Purification Methods
Purify it via the HgCl2 addition compound formed when phenanthridine (20g) in 1:1 HCl (100mL) is added to aqueous HgCl2 (60g in 3L), and the mixture is heated to boiling. The HgCl2 complex separates as yellow red crystals with m 195-198o [Arcus & Mesley J Chem Soc 1780 1953]. Conc HCl is then added until all of the solid has dissolved. The compound separates on cooling and is decomposed with aqueous NaOH (ca 5M). Phenanthridine is extracted into Et2O, evaporated, and the residue is crystallised from pet ether (b 80-100o) or EtOAc. [Cumper et al. J Chem Soc 45218 1962.] It is also purified by chromatography on activated alumina from *benzene solution, with diethyl ether as eluent. Evaporation of ether gives crystalline material which is freed from residual solvent under vacuum, then further purified by fractional crystallisation, under N2, from its melt. It was purified by zone melting and sublimes in a vacuum. The picrate has m 218.5-219.5o (from iso-PrOH) (also reported are m 244-245o and 247-248o, from EtOH or H2O). [Slough & Ubbelhode J Chem Soc 911 1957.] [Beilstein 20 H 466, 20 III/IV 4016, 20/8 V 223.]
Properties of o-Phenanthroline
| Melting point: | 114-117 °C(lit.) |
| Boiling point: | >330°C |
| Density | 1.1836 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| Flash point: | >330°C |
| storage temp. | Store below +30°C. |
| solubility | 2.69g/l |
| form | Powder |
| pka | 4.84(at 25℃) |
| color | White to light yellow or light pink |
| Water Solubility | slightly soluble |
| Sensitive | Hygroscopic |
| Merck | 14,7214 |
| BRN | 126461 |
| Stability: | Stable. Hygroscopic. Store under nitrogen. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 66-71-7(CAS DataBase Reference) |
| NIST Chemistry Reference | o-Phenanthroline(66-71-7) |
| EPA Substance Registry System | 1,10-Phenanthroline (66-71-7) |
Safety information for o-Phenanthroline
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for o-Phenanthroline
| InChIKey | DGEZNRSVGBDHLK-UHFFFAOYSA-N |
o-Phenanthroline manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 66-71-7 1,10-Phenanthroline 98%View Details
66-71-7 1,10-Phenanthroline 98%View Details
66-71-7 -
 1,10-Phenanthroline, 99% CAS 66-71-7View Details
1,10-Phenanthroline, 99% CAS 66-71-7View Details
66-71-7 -
 1,10-Phenanthroline CAS 66-71-7View Details
1,10-Phenanthroline CAS 66-71-7View Details
66-71-7 -
 1,10-Phenanthroline CAS 66-71-7View Details
1,10-Phenanthroline CAS 66-71-7View Details
66-71-7 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
